Abstract
Germline (GL) transcription is regulated by specific promoters and immunoglobulin heavy chain (IgH) 3′ locus enhancers and is necessary for Ig class-switch recombination (CSR). We have generated different transgenic lines containing the GL ϵ promoter, switch (S) ϵ region, and constant (C) ϵ region with or without the DNase I–sensitive regions (HS) 3A-HS1,2 or HS3B-HS4 3′ IgH enhancer pairs. The enhancerless construct was expressed in B cells activated by interleukin (IL)–4 and CD40, thus resembling regulation of the endogenous gene. Both enhancer-containing transgenes efficiently increased expression in B cells and were strongly up-regulated by stimuli. In addition, Sϵ regions of the transgene containing HS3B-HS4 were mutated in activated, sorted B cells. Such mutations are known to precede CSR and are dependent on activation-induced cytidine deaminase (AID). Our findings show that all elements necessary for recruitment of the recombination machinery are present in the transgene containing HS3 and HS4. These enhancers probably provide something more specific than mere increased accessibility of switch regions. We propose that transcription factors binding the enhancers help to target the recombination machinery to the switch regions.
Introduction
The immunoglobulin heavy chain (IgH) gene locus is a large gene cluster that is tightly regulated during B-cell development and differentiation. When a B cell is challenged by antigen, the IgH locus undergoes a diversification process, which affects both variable (V) and constant (C) genes. Point mutations, occasional insertions, and deletions are introduced into V regions by somatic hypermutation (SHM), allowing generation of high-affinity antibodies. Class-switch recombination (CSR) takes place in the C regions of the IgH locus and involves large repetitive switch (S) sequences that are located upstream of each CH gene segment. During CSR, the Sμ region recombines with a downstream S region, resulting in a closer proximity of the new CH gene (α, ϵ, or γ) segment to the V-diversity (D)–joining (J) region and the VH promoter. This enables B cells to express IgA, IgE, or IgG with various effector functions, but with retained antigen specificity.1,2
A B-cell–specific protein, activation-induced cytidine deaminase (AID), which is required for both SHM and CSR, was recently identified.3,4 It has been known for several years that nucleotide mutations (substitutions, deletions, and insertions), similar to those found in hypermutated Ig variable region, occur near S region recombination junctions.5 These mutations take place during CSR and are dependent on AID.6-8
CSR is preceded by activation of sterile germline (GL) transcripts of the CH gene, which later will be selected for recombination. Gene targeting experiments indicate that synthesis of GL transcripts is necessary for CSR.2 It was also shown that the level of GL transcription correlates with CSR efficiency.9 The GL transcription is driven by promoters located upstream of each S region. Different external stimuli activate or suppress GL promoters and CSR. For example, the GL ϵ promoter is induced by interleukin (IL)–4 plus lipopolysaccharide (LPS) or CD40 ligation, resulting in Ig class-switching to IgE.2
The IgH intronic (Eμ) enhancer and the IgH locus 3′ enhancers are required for efficient GL transcription and CSR. Deletion of Eμ impairs early events in B-cell differentiation (V to DJ rearrangement and μ-chain expression), but also reduces CSR.10-12 Four lymphoid-specific enhancers (DNase I–sensitive regions [HS] 3A, HS1,2, HS3B, and HS4, in this order) are located downstream of the murine IgH locus and have been claimed to be a locus control region (LCR).13,14 HS3A and HS3B are virtually identical but oriented opposite to each other.15,16 Except for HS4, which is functional from pre-B cells to plasma cells, this region is active in the late stages of B-cell differentiation (ie, during induction of GL transcripts, CSR, SHM, up-regulation of Ig expression, and secretion).13,17-19 The 3′ enhancers strongly up-regulate linked reporter gene expression in a B-cell–specific and position-independent manner.20-23 Published data indicate that they also are able to up-regulate transcription of GL promoters.24-26 The first evidence for involvement of the 3′ IgH enhancers in the regulation of GL transcription was obtained from a study where the HS1,2 enhancer was replaced by the neomycin gene and a heterologous promoter. It resulted in a severe reduction of GL transcription and CSR.27 However, so-called clean deletions of either HS1,2 or HS3A had no effect on GL transcription and CSR, demonstrating that the neomycin gene and/or its promoter interfered with these processes, and that individual enhancers are redundant.28 Interestingly, clean deletion of HS3B and HS4 together led to reduced levels of GL transcription and CSR of many Ig classes.29 In addition, when the IgH locus without the 3′ enhancers is expressed in artificial chromosomes in transgenic mice it results in severely reduced expression of GL transcripts and undetectable CSR.30 It remains unclear whether all enhancers contribute to up-regulation of GL transcription and induction of CSR. Deletion of different 3′ enhancers implies that the HS3B-HS4 enhancer pair might be the main player.29,31 On the other hand, deletion of only 1 enhancer (HS1,2 or HS3A) might be more easily compensated by other IgH locus enhancers.1
We wished to investigate a possible interaction between GL promoters and 3′ enhancers in a transgenic system and therefore made constructs, including the GL ϵ promoter, Iϵ, Sϵ, and Cϵ regions alone, or linked to either HS3A-HS1,2 or HS3B-HS4. We then analyzed expression of all 3 transgenes in different founders. We also investigated the question of whether Sϵ region mutations can be induced in the transgenic mice and if such mutations would be dependent on presence of the 3′ enhancers.
Materials and methods
Transgenic constructs
Epsilon–I-, S- C-region (EPS-ISC)
Mouse Iϵ, Sϵ, and Cϵ regions were amplified from genomic DNA and inserted into pBluescript II KS (+/−) phagemid vector (Stratagene, La Jolla, CA). The Iϵ fragment contained a −2057/+254 fragment, where +1 corresponds to the most upstream transcriptional start site.32 This corresponds to positions 68432-70739 in the mCG13669 sequence from the mouse Celera database.33 The Sϵ region contained all 20 units of tandem repeats and flanking sequences—a total of 1.3 kb, corresponding to positions 72369-73682 in mCG13669). The Cϵ region contained all 4 CH exons and the polyadenylic acid (polyA) site (74720-77065 fragment). The Cϵ region was amplified in 2 fragments and a tag sequence (36 bases: GAATTCCAGATCCTCCTCAGAAATCAGCTTTTGCTC) was inserted into the CH1 region to be able to distinguish transgenes from endogenous genes.34 Sequences for primers used in cloning can be obtained by request from the authors.
ENH (enhancer)–right and ENH-left constructs
Mouse HS3A-HS1,2 and HS3B-HS4 enhancers were amplified from a pCAT vector containing all IgH locus downstream enhancers (a kind gift from Dr M. Cogné, Limoges, France). The ENH-left vector included the EPS-ISC construct with attached HS3A-HS1,2 enhancers. The ENH-right vector contained the EPS-ISC construct and HS3B-HS4 enhancers. The HS3A and HS3B were included in a 2.1-kb EcoRI-HindIII genomic fragment, HS1,2 in a 0.6-kb StuI-EcoRV fragment, and HS4 in a 1.38-kb PstI-HindIII fragment.35 Additional information can be found in Document S1, available on the Blood website (see the Supplemental Materials link at the top of the online article). Schematic pictures of all constructs are shown in Figure 1.
Transgenic constructs and copy number. Schematic map of mouse IgH locus (top) with variable region exons (V, D, J) shown as □ and C region genes as ▪. Small ovals with arrows represent GL promoters, and larger ovals correspond to IgH locus enhancers. The 3 transgenic constructs are depicted below. The exons in transgenic constructs are represented as ⊡, and the tag sequence in CH1 is shown as ▧. The spliced transgenic transcript is shown below the EPS-ISC construct. The locations of RT-PCR primers used for Iϵ-CH1 and Iϵ-tag PCRs are indicated by arrows.
Transgenic constructs and copy number. Schematic map of mouse IgH locus (top) with variable region exons (V, D, J) shown as □ and C region genes as ▪. Small ovals with arrows represent GL promoters, and larger ovals correspond to IgH locus enhancers. The 3 transgenic constructs are depicted below. The exons in transgenic constructs are represented as ⊡, and the tag sequence in CH1 is shown as ▧. The spliced transgenic transcript is shown below the EPS-ISC construct. The locations of RT-PCR primers used for Iϵ-CH1 and Iϵ-tag PCRs are indicated by arrows.
Generation of transgenic mice
The constructs were cut out from the vector using BssHII restriction sites, purified, and used to generate transgenic founder lines. Positive founders were identified by polymerase chain reaction (PCR). Their offspring was analyzed for expression of transgenes. The sequences for primers used in typing can be found in Document S1.
Isolation of B cells and cell cultures
RNA extraction and reverse transcription (RT)–PCR
Total RNA was extracted and 1 μg was used to prepare cDNA using an oligo-dT primer. PCR was run to detect endogenous and transgenic GL ϵ transcripts. Mb-1 and γ-actin transcripts were used as controls.37 The Iϵ and CH1ϵ primer pair detected both endogenous and transgenic transcripts that could be distinguished by size. The Iϵ and Tag primer pair detected only transgenic transcript (Figure 1). Primer sequences can be found in Document S1.
Expression analysis by real-time PCR
Transgenic GL ϵ transcripts were quantified by real-time PCR using qPCR MasterMix Plus for SYBR Green I kit (Eurogentec, Seraing, Belgium) and by running samples on an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA). The Iϵ and Tag primers were used to amplify transgenic GL transcript. More details can be found in Document S1.
Determination of copy number by real-time PCR
Transgenic Sϵ region mutation analysis
Genomic DNA was prepared and amplified using the Expand High Fidelity PCR system (Roche, Indianapolis, IN). The EpsS-upp and Tag primers were used to amplify the transgenic Sϵ-CH1ϵ region, a 1.6-kb fragment. PCR product was cloned using the TOPO-TA cloning kit (Invitrogen, Carlsbad, CA) and sequenced using M13, T7 universal primers or an Sϵ region–specific primer (GGGCTGAACCAGATTGCACTA). The cloned fragment contained 1.3 kb of Sϵ region and 252 bases of C region (74720-74972 fragment from mCG13669). The S region included 990 bases of tandem repeats, 224 bases 5′, and 188 bases 3′ of the repeats. For analysis of transgenic Iϵ region, the upstream primer (CCACCCCACTTTTAGCTGAGG) hybridized to the −55 to −36 region of the GL ϵ promoter and the downstream primer (CTTAGCCCAGTCTCTCGAGAA) hybridized to the junction of Iϵ and Sϵ in the transgenic construct, but not to the endogenous region. A region of 330 nucleotides was thereby amplified. The region from the most upstream start site of transcription32 to the end of the I region present in the construct was analyzed for mutations. Sequencing was performed by MWG-Biotech AG (Ebersberg, Germany). Sequence alignments were performed using DNASTAR Lasergene software (Madison, WI).
Results
Expression of GL ϵ transgenes
We prepared a construct containing the Iϵ promoter, the Iϵ exon, 1.3 kb of the Sϵ region, and the Cϵ region. A 36-base tag sequence was inserted into the CH1 region, which enabled us to distinguish the transgene from endogenous gene by size or using a specific PCR primer. Both splice sites surrounding the Sϵ region were also included to ensure proper expression and processing of the transgene. The so-called EPS-ISC construct, the derived RNA, and the RT-PCR primers are schematically shown in Figure 1.
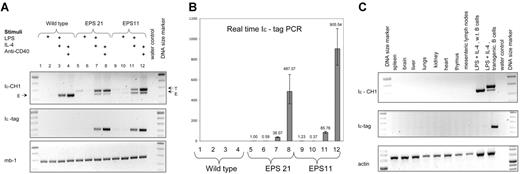
Two different transgenic lines of EPS-ISC were analyzed: EPS11 and EPS21, with approximately 24 and 15 copies of the transgene, respectively (Table 1). First, we investigated expression of the transgenes in nonactivated or activated B cells. Conventional RT-PCR did not show any expression of the transgene in nonstimulated or day-2 LPS–stimulated B cells (Figure 2A) However, real-time RT-PCR showed a detectable signal in both these groups compared with that in controls without template. Interestingly, LPS suppressed expression of the EPS-ISC transgene (Figure 2B). No expression of the endogenous GL ϵ transcripts was detected by conventional RT-PCR or real-time RT-PCR in nonstimulated or LPS-stimulated B cells.
Expression of the EPS-ISC transgene is B cell specific and induced by stimuli. (A) RT-PCR was performed with RNA extracted from enriched wild-type B cells (lanes 1-4), EPS21 (lanes 5-8), or EPS11 (lanes 9-12) transgenic mice after 2 days of culture in the presence of indicated stimuli. In samples, which were amplified with Iϵ and CH1 primers, the top band corresponds to the transgenic transcript (T), and the bottom band to the endogenous (E) GL ϵ transcript. Only the transgenic band is detected in the Iϵ-tag PCR. The Mb-1 RT-PCR was used for cDNA quality control. (B) RNA expression from the same experiment was quantified by real-time RT-PCR. All samples were compared with the noninduced EPS21 sample. The y axis shows relative expression. Mean values and SD of triplicates are shown. (C) RT-PCR was performed with RNA extracted from different organs of an EPS21 transgenic mouse. The γ-actin transcript was used to control for cDNA quality.
Expression of the EPS-ISC transgene is B cell specific and induced by stimuli. (A) RT-PCR was performed with RNA extracted from enriched wild-type B cells (lanes 1-4), EPS21 (lanes 5-8), or EPS11 (lanes 9-12) transgenic mice after 2 days of culture in the presence of indicated stimuli. In samples, which were amplified with Iϵ and CH1 primers, the top band corresponds to the transgenic transcript (T), and the bottom band to the endogenous (E) GL ϵ transcript. Only the transgenic band is detected in the Iϵ-tag PCR. The Mb-1 RT-PCR was used for cDNA quality control. (B) RNA expression from the same experiment was quantified by real-time RT-PCR. All samples were compared with the noninduced EPS21 sample. The y axis shows relative expression. Mean values and SD of triplicates are shown. (C) RT-PCR was performed with RNA extracted from different organs of an EPS21 transgenic mouse. The γ-actin transcript was used to control for cDNA quality.
LPS plus IL-4 or anti-CD40 plus IL-4 strongly enhanced transgene expression. LPS plus IL-4 increased expression 150 to 200 times and anti-CD40 plus IL-4 raised the response 1200 to 1500 times in the EPS21 transgenic line. The EPS11 transgenic line showed even higher expression (2000-3000 times) after stimulation with anti-CD40 plus IL-4, possibly indicating a copy-number dependence. On a per-gene basis, RNA expression from the induced EPS-ISC transgene was approximately 30% of the endogenous gene (Figure S1). This implies that additional regulatory elements are needed for full expression.
We also investigated expression of the EPS-ISC transgene in different organs (Figure 2C). No expression was detected in any tissue except activated B cells. Thus, the EPS-ISC transgene shows similar pattern of expression as the endogenous GL ϵ gene.
Expression of GL ϵ transgenes linked to 3′ enhancers
Expression of the GL γ2a promoter linked to an HS1,2-HS3B-HS4 enhancer cassette,24 and the GL γ2b promoter linked to an HS3A-HS1,2-HS3B-HS4 enhancer cassette (our unpublished data, March 2003) are B-cell specific, but are not regulated by appropriate stimuli. Therefore, we chose to investigate GL ϵ promoter regulation by IgH locus 3′ enhancers by linking the EPS-ISC construct to either the HS3A-HS1,2 or the HS3B-HS4 enhancer pair (Figure 1). Three different lines of transgenic animals were analyzed for each construct. The ENH-right lines contained the EPS-ISC construct linked to HS3B-HS4, and ENH-left lines included EPS-ISC linked to HS3A-HS1,2. Copy numbers for each line are shown in Table 1.
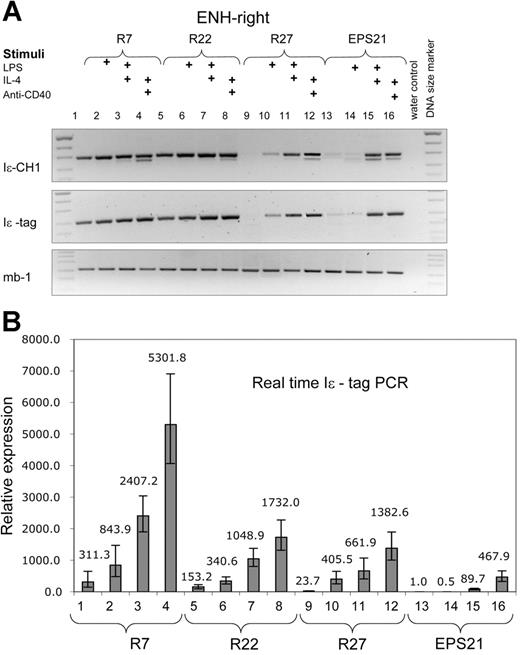
Both constructs showed enhanced transcription compared with the EPS-ISC. The ENH-right transgenic lines (R7, R22, and R27) exhibited higher expression in nonstimulated cells compared with that of the ENH-left lines (Figures 3-4). Expression correlated with the copy number of the transgenes in nonstimulated cells. However, the copy number dependence was less pronounced in activated B cells. The level of transgene expression after IL-4 plus anti-CD40 stimulation was similar for founder R27 with 3 copies, and founder R22 with 14 copies. The 3 ENH-right transgenic lines show integration site independence.
The ENH-right transgene expression is higher than that of the enhancer-less construct and is induced by cytokines. RT-PCR was performed with RNA from 3 different transgenic ENH-right lines (R7, lanes 1-4; R22, lanes 5-8; and R27, lanes 9-12) and the EPS21 transgenic line (lanes 13-16). The experiment is performed in the same manner as in Figure 2. (A) Results from RT-PCR. (B) The same samples are quantified by real-time RT-PCR. All samples are compared with nonstimulated cells of EPS21.
The ENH-right transgene expression is higher than that of the enhancer-less construct and is induced by cytokines. RT-PCR was performed with RNA from 3 different transgenic ENH-right lines (R7, lanes 1-4; R22, lanes 5-8; and R27, lanes 9-12) and the EPS21 transgenic line (lanes 13-16). The experiment is performed in the same manner as in Figure 2. (A) Results from RT-PCR. (B) The same samples are quantified by real-time RT-PCR. All samples are compared with nonstimulated cells of EPS21.
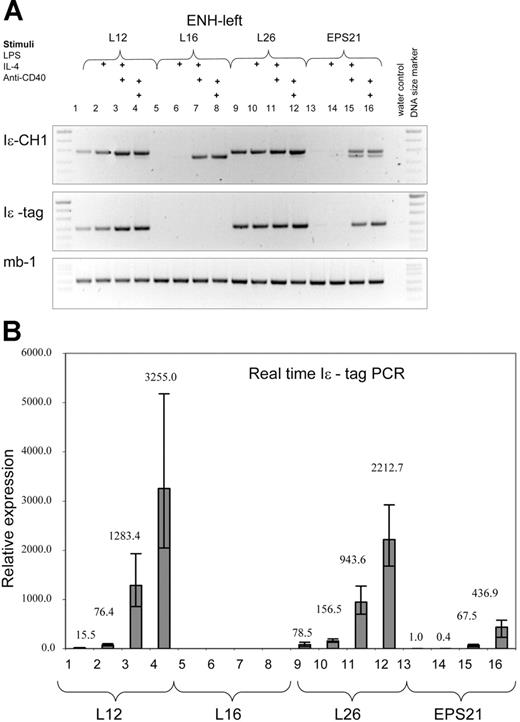
The ENH-left transgene expression is comparable to that of the ENH-right transgene. RT-PCR was performed with RNA from 3 different transgenic ENH-left lines (L12, lanes 1-4; L16, lanes 5-8; and L26, lanes 9-12) and the EPS21 line (lanes 13-16). See legend to Figure 3 for explanations.
The ENH-left transgene expression is comparable to that of the ENH-right transgene. RT-PCR was performed with RNA from 3 different transgenic ENH-left lines (L12, lanes 1-4; L16, lanes 5-8; and L26, lanes 9-12) and the EPS21 line (lanes 13-16). See legend to Figure 3 for explanations.
We have analyzed 3 ENH-left transgenic lines (L12, L16, and L26). Expression was integration site dependent, since we could not detect transcription in B cells from founder L16, which harbored 2 copies of the construct. The other 2 lines, L12 (13 copies) and L26 (approximately 45 copies), showed weak expression in nonstimulated cells. LPS gave rise to higher transgene expression compared with that of nonstimulated cells. However, the increase was lower (150-300 times) than that of the corresponding group in the ENH-right transgene (800-2000 times). IL-4 plus LPS or anti-CD40 further induced the transgenic GL ϵ promoter, reaching similar levels of transcription as those of the ENH-right transgenic lines.
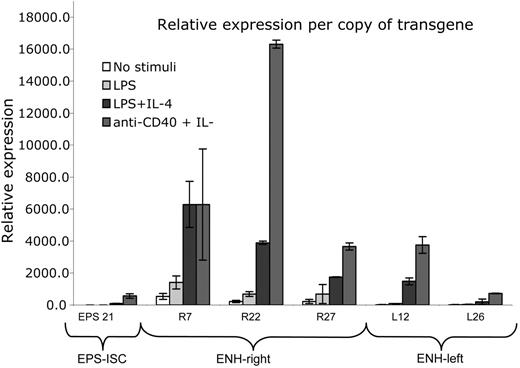
In Figure 5, we compared transgene expression on a per-gene basis. A summary of several experiments showed that the ENH-right transgene was expressed more efficiently in nonstimulated and LPS-stimulated cells compared with that in the other 2 transgenic lines. The level of expression of both enhancer-containing transgenes was copy number independent. There was no significant difference in the LPS plus IL-4 or anti-CD40 plus IL-4 responses comparing R7 or R27 with L12. However, the R22 transgene was expressed at a higher rate than was L12 after stimulation with LPS plus IL-4 or anti-CD40 plus IL-4 (Figure 5 legend).
Expression level on a per-gene basis in ENH-right and ENH-left constructs is integration site dependent and higher than that of the EPS-ISC construct. The data represent a summary of different real-time RT-PCR experiments, analyzed on a per-gene basis. The RNA expression in nonstimulated B cells from the EPS21 transgenic line was considered to be 1, and all samples from different transgenic lines and stimulation conditions were compared to it. The average values from 6 independent experiments (EPS21 line) or 2 independent experiments (all other transgenic lines) are shown and SDs are indicated. When compared with EPS-ISC, the stimulated responses of R7, R22, R27, and L12, but not L26, were significantly different (P < .05 or lower). The LPS plus IL-4 or anti-CD40 plus IL-4 responses of R7 and R27 were not significantly different from that of L12, but responses in R22 were (P < .01 and P < .001, respectively).
Expression level on a per-gene basis in ENH-right and ENH-left constructs is integration site dependent and higher than that of the EPS-ISC construct. The data represent a summary of different real-time RT-PCR experiments, analyzed on a per-gene basis. The RNA expression in nonstimulated B cells from the EPS21 transgenic line was considered to be 1, and all samples from different transgenic lines and stimulation conditions were compared to it. The average values from 6 independent experiments (EPS21 line) or 2 independent experiments (all other transgenic lines) are shown and SDs are indicated. When compared with EPS-ISC, the stimulated responses of R7, R22, R27, and L12, but not L26, were significantly different (P < .05 or lower). The LPS plus IL-4 or anti-CD40 plus IL-4 responses of R7 and R27 were not significantly different from that of L12, but responses in R22 were (P < .01 and P < .001, respectively).
It would appear from the experiments shown in Figures 2 and 3 that the endogenous GL ϵ transcripts are differently expressed in the 3 transgenic strains. However, this is most likely due to primer competition. In Figure S1, we have compared endogenous GL ϵ transcription in relation to Mb-1 expression in the 3 transgenic strains and, as shown, the expression is very similar. In addition, the levels of transgenic GL ϵ transcripts are compared with that of Mb-1 to allow a direct comparison between endogenous and transgenic GL ϵ transcripts. In cells stimulated by LPS plus IL-4 or anti-CD40 plus IL-4, expression of the transgene in ENH-right was 2- to 10-fold higher than that of the endogenous gene on a per-gene basis. A corresponding comparison showed a 2- to 5-fold higher expression in ENH-left transgenics (Figure S1).
Taken together, our data reveal that both HS3A-HS1,2 and HS3B-HS4 enhancer pairs increase transcription from the linked GL ϵ gene very efficiently. There was no substantial difference in expression of ENH-right and ENH-left transgenic constructs in stimulated B cells, although the ENH-right transgene had higher activity in nonstimulated and LPS-stimulated cells. We have measured steady-state levels of transcripts on day 2 of activation. Thus, we cannot exclude that there is a difference in transcription rate comparing the 2 enhancer-containing strains. We find this unlikely, since appearance of the transcripts is similar in all 3 strains (data not shown) and the rate of degradation is not likely to be different.
Expression of the ENH-right and ENH-left transgenes was evaluated in different organs and was detected by RT-PCR in spleen and mesenteric lymph nodes, and weakly in lungs. It correlated with the presence of Mb-1 RNA, indicating that it reflected presence of B cells (data not shown).
Evaluation of transgenic Sϵ region mutations
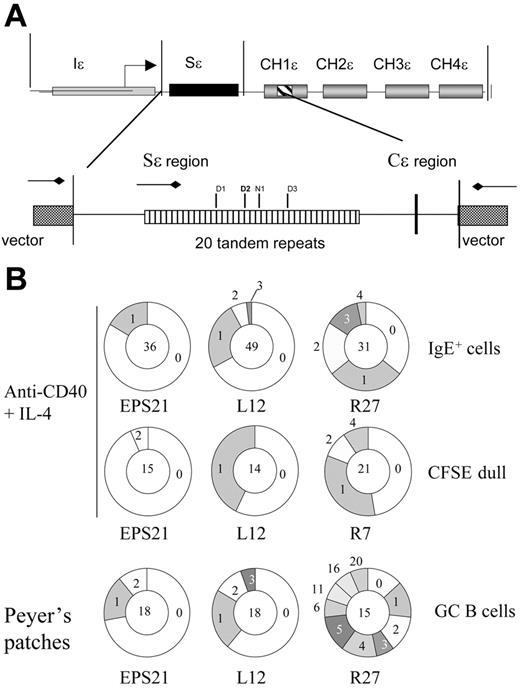
It has been shown that mutations in the Sϵ region are controlled by AID and are associated with CSR.5-8 Therefore, we investigated whether induction of mutations occurred in transgenic S regions from EPS-ISC, ENH-left, and ENH-right mice. B cells stimulated for 3 to 6 days were analyzed as detailed in “Materials and methods.” No significant difference in S region mutation frequency was found, comparing DNA extracted from activated B cells with tail DNA or comparing enhancerless transgenes to ENH-right or ENH-left (mutation frequency in B-cell DNA, 2.5-5.8 × 10−4; in tail DNA, 5.8 × 10−4). We then tested sorted IgE+ cells from B cells activated for 6 days with anti-CD40 plus IL-4 and found that the number of Sϵ-region mutations in the ENH-right transgene was significantly elevated (13 × 10−4) compared with that of enhancerless transgenes (3 × 10−4) or tail DNA (Table 2, Experiment 1). Interestingly, the mutation frequency of the transgenic I region was even higher than that of the S region (17 × 10−4), whereas the transgenic C-region in ENH-right was not significantly elevated. Also, there appeared to be a difference in mutation rate in different parts of the S region, such that the 5′ or the middle part had higher frequency of mutations than the 3′ part of the S region (Table 2 and Figure S2). The number of mutations in the ENH-left transgenic S regions was twice that of the enhancerless transgene, a significant difference. However, it was not significantly different from tail DNA. These experiments were repeated twice, also using the L26 and R22 founders, with similar results (data not shown).
In vitro–activated IgE+ cells have generally divided more times than cells expressing IgM or IgG.40 We therefore analyzed mutation frequencies in cells, which had divided many times. Spleen B cells were labeled with CFSE and cultured with anti-CD40 plus IL-4. On day 6, the 10% dullest cells were isolated by sorting and analyzed as detailed previously. Again, the frequency of Sϵ region mutations was significantly higher in the ENH-right transgene (founder R7), but not in enhancerless or ENH-left transgenes (Table 2, Experiment 2).
Since Sμ regions are highly mutated in germinal center (GC) B cells from Peyer patches,41 we tested to see if the transgenic Sϵ regions were mutated in this population. CD45R(B220)+ PNAhigh cells from Peyer patches were sorted and transgenic Sϵ regions were analyzed. As shown in Experiment 3 of Table 2, the ENH-right (founder R27) transgenic mice exhibited much increased mutation frequency, 47 × 10−4, compared with that of the enhancerless transgenes (5.7 × 10−4). We also tested another founder of ENH-right (founder R7) and found that the mutation frequency of GC B cells was lower (18 × 10−4), but still significantly elevated compared with the controls (Table 2, Experiment 3). The mutation frequency of transgenic Sϵ regions of ENH-left mice was not significantly different from that of either tail DNA or enhancerless transgenes.
We also investigated the types of mutations. Most mutations were in the form of single-nucleotide substitutions. Interestingly, the number of mutations at G or C residues was generally higher in ENH-right transgenes (45%-75%) compared with the other 2 transgenic strains (< 17%-50%). Intra-S region deletions have been shown to be AID dependent and to be increased after activation of B cells.42 Deletions were very rare in transgenic Sϵ regions of EPS-ISC or ENH-left mice, but were repeatedly observed in in vitro–activated cells from ENH-right mice (Table 2, Figure S2). The deletions were most often occurring within 1 Sϵ region, but occurred at least once between 2 Sϵ regions (Figure S2). The spectrum of mutations from the ENH-right transgenic Sϵ region is shown in Figure S2 and Figure S3. Interestingly, whereas mutations in in vitro–activated B cells were concentrated to the 5′ region, they occurred throughout the analyzed Sϵ region in GC B cells. Furthermore, in the latter, deletions were more rare. Analysis of the number of mutations at WRCY/RGYW, which are hotspots for endogenous V and S region mutations,43 did not reveal any marked difference comparing the different strains (50%-71% of mutations at hot spots with all transgenes).
The numbers of mutations per clone for the experiments shown in Table 2 are given in Figures 6B and Figure S4. As shown, the numbers of unmutated clones are in the majority in enhancerless and ENH-left transgenes. The former had no clone above 2 mutations, whereas the ENH-left transgene had 3 mutations per clone at most. Clones from in vitro–activated ENH-right transgenic mice had 4 mutations per clone at most, but in GC cells of Peyer patches there were as many as 20 mutations per clone. The mutation frequency and the numbers of mutations per clone in this cell population reach observed values in endogenous V genes.41
Sϵ mutation analysis. (A) Schematic map of the transgenic ϵ construct and Sϵ region that was used to evaluate the mutation frequency. The reported recombination sites are indicated within the tandem repeats. Sites D1 to D3 are reported by Dunnick et al,5 and site N1 is reported by Nikaido et al.44 (B) Pie charts depicting the proportion of sequences that carry 0, 1, 2, 3, etc mutations over the 681-bp region analyzed. The number of clones analyzed is shown in the middle. Clones with identical mutations were counted only once. The experiments are the same as the ones shown in Table 2.
Sϵ mutation analysis. (A) Schematic map of the transgenic ϵ construct and Sϵ region that was used to evaluate the mutation frequency. The reported recombination sites are indicated within the tandem repeats. Sites D1 to D3 are reported by Dunnick et al,5 and site N1 is reported by Nikaido et al.44 (B) Pie charts depicting the proportion of sequences that carry 0, 1, 2, 3, etc mutations over the 681-bp region analyzed. The number of clones analyzed is shown in the middle. Clones with identical mutations were counted only once. The experiments are the same as the ones shown in Table 2.
We conclude that transgenic Sϵ regions of 2 different founders of ENH-right mice can be induced in B cells in vivo or in vitro to mutate to a significantly higher frequency compared with the enhancerless transgenic strain. Although it appeared that B cells from ENH-left transgenics had slightly elevated mutation rates compared with that of the enhancerless strain, these differences were not always significant.
Discussion
Our data obtained from EPS-ISC transgenic mice indicate that 2 kb of the GL ϵ promoter region contains all necessary elements for B-cell–specific, properly regulated expression of GL ϵ transcripts. However, on a per-gene basis, the induced transcription of the EPS-ISC transgene was roughly 30% of that of the endogenous gene. Both founders contained relatively high copy numbers and it is possible that repeated promoters interacted and synergized, leading to increased transcription. In conclusion, our data, together with published results,24,30 indicate that additional regulatory elements are needed to obtain efficient GL transcription.
Both HS3A-HS1,2 and HS3B-HS4 up-regulated the linked GL ϵ promoter to a similar extent in stimulated cells. Although LPS suppressed EPS-ISC transcription, it induced both enhancer-containing transgenes. This finding implies that the synergy observed between LPS and IL-4 in the endogenous locus is in part provided by presence of the enhancers. Thus, it is likely that in the endogenous locus IL-4 is acting on the GL ϵ promoter and LPS on both the ϵ promoter and the 3′ enhancers or only on the enhancers. Stimulation with IL-4 plus LPS or anti-CD40 dramatically enhanced GL ϵ transcriptions. All 3 transgenic constructs showed the same pattern of regulation as the endogenous gene, being induced by IL-4 plus LPS and showing highest activity after IL-4 plus anti-CD40 stimulation. Background transcription of enhancer-containing constructs was clearly higher than that of the endogenous gene. However, it could not be compared on a per-gene basis since we were not able to detect expression of the endogenous gene in nonstimulated cells.
None of our constructs and founders showed silencing of the transgene with increased age or generation, in contrast to other transgenes that included IgH regulatory elements.23,45 In these studies xenogenous elements (green fluorescent protein [GFP]) were included in the transgenic constructs. Such sequences can cause silencing, as has been demonstrated.46
We observed a correlation between gene expression and copy number in nonstimulated B cells, whereas no correlation was observed when cells were stimulated. All 3 ENH-right transgenic lines, but only 2 of 3 lines of ENH-left mice, expressed the transgene. This indicates integration site dependence of transcription in the latter mice. Thus, none of our transgenes possessed LCR properties.
We also investigated involvement of 3′ enhancers in CSR by analyzing induction of mutations in the transgenic Sϵ region. Small deletions, nucleotide substitutions, and insertions are introduced in S regions of IgH genes before CSR.5 Such mutations preferentially occur in the Sμ region, but can sometimes be detected in Sγ regions or around Sμ/Sγ junctions.7,8,42,47,48 These mutations are AID dependent and induced by the same stimuli that also induce CSR. Involvement of IgH 3′ enhancers in this process has not previously been addressed. We have sequenced transgenic Sϵ regions from each transgenic construct after 4 to 6 days of culture with or without stimuli, but did not observe an increased mutation frequency. However, when in vitro–activated IgE+ or highly proliferating B cells were analyzed, we detected a significantly increased frequency of Sϵ region mutations in ENH-right transgenic lines. Although the transgenes were present in several copies, only the I and S regions, but not the C region, had increased mutations, indicating similar specificity as for endogenous S regions.
We observed a higher mutation frequency in the Iϵ region of the ENH-right transgenes. Xue et al recently found that I region mutations are inducible and start around 150 nucleotides downstream of the exon start site.49 Except for Iμ, these regions are deleted after CSR. We observed no increased mutation rate in the Cϵ region, but more mutations were found in the 5′ Sϵ region than the 3′ region. Similar observations were done by Xue et al in analysis of Sμ, Sγ1, and Sγ3 regions.49
Our data indicate that mutations occur in a subset of activated B cells. They are in line with those of Reina-San-Martin et al,47 who showed that Sμ mutations are preferentially induced in cells that have divided several times. We have not been able to detect any trans-switching between endogenous Sμ and transgenic Sϵ, making the possibility less likely that this is the reason for induced mutations in IgE+ cells (data not shown). It might instead be due to the fact that IgE+ cells generally have divided more times than cells producing other Ig classes.40 The fact that GC B cells from Peyer patches of ENH-right transgenes had highly increased Sϵ mutation frequency confirms this interpretation. This population is enriched for IgA-producing cells, whereas the percentage of IgE+ cells probably is much lower. Also, this population is continuously activated by intestinal pathogens and has presumably been dividing several times.
There was no indication of Sϵ mutations in enhancerless transgenes in any condition, although the transgene was properly transcribed. ENH-left transgenes were transcribed at similar steady-state levels as ENH-right transgenes, but the mutation frequency of transgenic Sϵ regions was in most experiments not significantly different from that of controls. It has been suggested that the structure of S regions is important for CSR.50 According to 1 model, R-loops are formed by transcription over S regions, leaving 1 DNA strand accessible for attack by the recombination machinery.51 Another model propose that G-loops formed in transcribed S-regions has a similar function.52 Similar structures are probably formed with all of our transgenes, indicating that although they might be necessary, they are not sufficient to induce recombination.
Requirement for the presence of enhancers for induction of SHM in V genes was observed already 1994 by Betz et al.53 In this study, different parts of the κ locus were included in transgenes. It was shown that the Vκ promoter could be replaced but the intron and 3′ Eκ enhancers were both required for full SHM of Vκ. In part, this was correlated with increased levels of transcription, emphasizing its importance. In contrast, van der Stoep et al showed that genomic clean deletion of the 3′ Eκ did not perturb the Vκ mutation rate.54 This probably demonstrates redundancy among the 2 Eκ enhancers but also illustrates the discrepancy in results using transgenic versus knockout technology. One limitation with the former is that the structure of the transgene is altered, which might influence the results.54 In the present study, we compare 2 different IgH enhancer-containing transgenic strains that are both transcribed at high efficiency and find that Sϵ regions in only 1 type of transgene are mutated at significantly higher rates than the controls. Thus, there is no obvious relation between transcription level and mutation rate comparing the 2 enhancer-containing strains. However, we cannot exclude that ENH-left transgenes do not mutate due to structural constraints of the transgene. Collectively, our mutation data indicate that recruitment of the recombination machinery has more specific requirements other than high levels of transcription or accessibility. The most straightforward explanation is that specific transcription factors or coactivators binding to HS4 or the combination of HS3 and HS4 are necessary and sufficient for recruitment of the recombination machinery. Alternatively, the HS1,2 enhancer might contain elements which inhibit mutations.
Peters and Storb55 found that somatic hypermutation only occurs within a certain distance from the V promoter. They proposed that a mutator is associated with the transcription complex. After a certain distance, the mutator is disassembled from the complex. We suggest that the function of IgH 3′ enhancers could be to specifically load the AID-dependent recombination machinery onto the complex. This is similar to what Xue et al49 recently proposed. In line with this, Shimizu and coworkers have shown that AID is coprecipitated together with RNA polymerase II.56 Furthermore, the fact that selected expressed genes are mutated in T cells of AID transgenic mice and in a B-cell lymphoma expressing AID is in favor of this hypothesis.57,58 These genes might in part be controlled by the same transcription factors as those regulating GL transcripts.
Terauchi et al demonstrated that the presence of HS3B and HS4 can induce VH gene mutations in a transgene also containing the intron enhancer,59 showing the importance of these regions for somatic hypermutation. However, the regions are clearly dispensable, since Le Morvan et al have shown that HS3B and HS4 knockout mice could undergo affinity maturation and VH gene mutations at levels indistinguishable from that of control mice.60 As discussed previously, the different results could be related to the different technologies used. However, our data using transgenics are in agreement with those of Pinaud et al29 using knockout strategy, that HS3 and HS4 are important for CSR.
We have shown that correct expression of the GL ϵ promoter in context of chromatin does not require the presence of IgH 3′ enhancers. However, both pairs (HS3A-HS1,2 and HS3B-HS4) of enhancers efficiently increase expression of the GL ϵ promoter. Our data, together with those of Pinaud et al,29 indicate a specific role for HS4 or a combination of HS3 and HS4 for inducing CSR. This is the first time a transgene of the IgH locus has been shown to be regulated in a similar way as the endogenous gene, with regard to GL transcription and S region mutations. Thus, this mouse strain will provide an important tool for future studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: J.L. and E.S. designed the study and carried out the research; J.L., V.T., and E.S. analyzed the data and wrote the paper.
Acknowledgments
We thank Dr M. Cogné for providing the enhancer cassette containing plasmid and for advice, Drs. Tasuku Honjo and Cristina Rada for advice, the Transgenic Animal Core facility of the Department of Cell and Molecular Biology at the Karolinska Institutet for preparation and care of transgenic mice, Birgitta Wester for help with cell sorting, Daniela Hahn for help with certain experiments, and Lena Ström for critically reading the manuscript.
This work was supported by the Swedish Research Council, the Vårdal Foundation, the Swedish Society for Medical Research (SSMF), the Royal Swedish Academy of Sciences, and the Karolinska Institutet.