Abstract
Churg-Strauss syndrome (CSS) is a systemic disease that shows marked eosinophilia along with eosinophil infiltration in the tissue. Prolonged eosinophil survival plays an important role in the pathogenesis of CSS; however, its detailed molecular mechanism remains unclear. Discoidin domain receptor 1 (DDR1) is a receptor tyrosine kinase, and its ligand is collagen. DDR1 was expressed in human leukocytes and fibroblasts, and it plays an important role in leukocyte cytokine production and fibroblast survival in an NF-κB–dependent manner. In this study, we examined in vitro and in vivo eosinophil DDR1 expression and its function in CSS patients. The expression level of DDR1 was significantly higher in the eosinophils of CSS patients, and the predominant isoform was DDR1b. Immunohistochemical findings revealed that the tissue-infiltrating eosinophils expressed endogenous DDR1. In CSS patients, DDR1 activation inhibited Fas agonistic antibody–induced apoptosis and up-regulated Fas agonistic antibody–induced cytokine production of eosinophils in an NF-κB–dependent manner. Suppression of DDR1 expression in the eosinophils by using RNA interference and addition of the DDR1-blocking protein abolished these effects. We propose that DDR1 contributes to the eosinophil survival in the tissue microenvironment of CSS and that it might be involved in the development of CSS.
Introduction
Churg-Strauss syndrome (CSS) is a rare disorder that is characterized by asthma, hypereosinophilia, and evidence of vasculitis with massive eosinophil infiltration affecting a number of organs.1 Infiltrating eosinophils are frequently detected in granulomatous lesions,2 and the fraction of eosinophils in the peripheral blood correlates with the course of the disease,2,3 suggesting possible eosinophil involvement in the pathogenesis of CSS.4 Peripheral and tissue eosinophilia is the hallmark of CSS,1,5 and prolonged eosinophil survival has been reported in CSS.6 However, the detailed mechanism underlying prolonged eosinophil survival in the tissue microenvironment has not been completely elucidated.
Discoidin domain receptor 1 (DDR1) is a receptor tyrosine kinase that is activated on binding to its ligand—collagen7,8 —a component of the extracellular matrix (ECM) in the organs. DDR1 possesses a unique extracellular domain that is homologous to discoidin 1 of Dictyostelium discoideum.9 DDR1 is constitutively expressed in the normal tissues of organs such as the lungs, kidneys, colon, and brain as well as in the tumor cells of epithelial origin, such as those of mammary, ovarian, and lung carcinomas.9 Five DDR1 isoforms (a, b, c, d, and e) can be generated by alternative splicing of the DDR1 gene, and only 2 isoforms (DDR1a and DDR1b) have been reported to be functional.10-15 We have previously reported that in vitro DDR1 expression could be induced in human leukocytes.13 The in vivo tissue-infiltrating leukocytes express DDR1 also.16 We discovered that DDR1 activation up-regulates the production of chemokines in an NF-κB–dependent manner and is likely to contribute to the development of inflammatory responses in the tissue microenvironment.10 In addition, we have recently found that DDR1 conferred protection to the pulmonary fibroblasts from Fas ligand–induced apoptosis.11 These findings led us to hypothesize that DDR1 might contribute to the prolonged survival of eosinophils in CSS.
In this study, we examined DDR1 expression in the eosinophils of CSS patients and asthma patients. We also investigated the antiapoptotic effect of DDR1. The following results were obtained: The eosinophils of CSS patients expressed endogenous DDR1; the DDR1b isoform was predominantly expressed; and the DDR1 activation conferred protection against Fas (CD95) agonistic antibody–induced apoptosis of eosinophils in an NF-κB–dependent manner. The tissue-infiltrating eosinophils expressed DDR1. We suggest a possible contribution of DDR1 in prolonging eosinophil survival in CSS.
Patients, materials, and methods
Patients
This study was reviewed and approved by the Kagoshima University Faculty of Medicine Committee on Human Research. We investigated 18 patients with CSS who were admitted to the Division of Respiratory Medicine, Respiratory and Stress Care Center, Kagoshima University Hospital, from 1995 to 2005. The patient group comprised 8 males and 10 females, and the mean age was 58.2 ± 18.2 years (mean ± standard deviation). For comparison, we also investigated 19 patients with asthma. The male-female ratio was 8:11 and the mean age was 58.9 ± 15.3 years (as diagnosed by the physician according to the National Asthma Education and Prevention Program Expert Panel Report 217 ). We also recruited 12 healthy volunteers; the male-female ratio was 5:7 and the mean age was 58.9 ± 13.1 years. All the patients and volunteers submitted their informed written consent prior to participating in the study. CSS was diagnosed according to the 1990 edition of CSS diagnosis criteria published by the American College of Rheumatology.2 All patients presenting with CSS fulfilled more than 5 criteria. We excluded patients with rheumatoid arthritis, diabetes mellitus, acute or chronic liver disease, and immunologic abnormalities that predispose to opportunistic infections.
Preparation of eosinophils
Human eosinophils were isolated from heparinized venous blood. Heparinized venous blood was mixed with one-fourth volume of 2% dextran solution (Sigma-Aldrich, St. Louis, MO) to precipitate the red blood cells. After 30 minutes of incubation at room temperature, the leukocyte-rich plasma was layered onto Histopaque and centrifuged at 800g for 20 minutes at room temperature. The granulocytes were separated from the erythrocytes by lysis in 0.2% NaCl, washed in phosphate-buffered saline (PBS) 3 times at 4°C, and the eosinophils were then isolated by negative selection using magnetic beads (eosinophil isolation kit, Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. The eosinophils were resuspended in RPMI 1640 containing 10% FCS and streptomycin/penicillin (complete medium). The purity of eosinophils was more than 99% according to a morphologic examination after staining with Diff-Quick (Wako, Tokyo, Japan).
Flow cytometry analysis
To detect the expression of DDR1 on eosinophils, 5 × 105 cells were collected after incubation in various states. The cells were washed 3 times with PBS and then incubated with human serum (sample collected from a healthy volunteer) for 10 minutes and subsequently incubated with FITC-conjugated anti–human monoclonal DDR1 antibody (48B3; Santa Cruz Biotechnology, Santa Cruz, CA) for 20 minutes at 4°C. After washing 3 times with PBS, flow cytometry analysis was performed by a FACScan using CellQuest software (PharMingen, San Diego, CA).
Production of DDR1-blocking protein
Wild-type cDNA of human DDR1b was kindly donated by Dr Teizo Yoshimura (Laboratory of Molecular Immunoregulation, National Cancer Institute at Frederick, MD). The N-terminal coding region of DDR1 (N-DDR1: 142 bp to 1389 bp) includes the collagen-binding site, while its C-terminal coding region (C-DDR1: 1390 bp to 2884 bp) does not include the collagen-binding site; these regions were amplified from the above-mentioned cDNAs using polymerase chain reaction (PCR) (1 cycle at 94°C for 5 minutes; 30 cycles at 94°C for 1 minute, at 62°C for 1 minute, and at 72°C for 1 minute) with LA Taq polymerase in a Gene Amp PCR System 9700 thermal controller (PE Applied Biosystems, Foster City, CA) using the following primers: sense: N-DDR1F, BamHI (5′-GCGGGATCCATGGGACCAGAGGCCCTGTCA-3′) and antisense: N-DDR1R, EcoRI (5′-GGGGAATTCGGGCTCCCCTCGGCCTTGGC-3′); sense: C-DDR1F, BamHI (5′-GCGGGATCCGACCGCCATCCTCATCGGCTG-3′) and antisense: C-DDR1R, EcoRI (5′-GGGGAATTCCACCGTGTTGAGTGCATTCT-3′) (underlined segments indicate restriction enzyme recognition sites). The PCR products were electrophoresed on agarose gels, excised, purified using QIAquick Gel Extraction Kit (Qiagen, Valencia, CA), digested with BamHI and EcoRI, and ligated into the BamHI and EcoRI sites of the pCMV Tag4 vector, which includes the Flag-coding region (Stratagene, La Jolla, CA). The constructs were sequenced on both strands at least twice to verify the sequence using the ABI PRISM310 autosequencer (PE Applied Biosystems).
The HEK-293 cells were maintained in DMEM containing 10% FBS, 2 mM l-glutamine, 50 U/mL penicillin G, and 50 μg/mL streptomycin. The parental HEK-293 cells were transfected with either a vector containing the N-DDR1 coding region or a vector containing the C-DDR1 coding region by using the Effectene transfection reagent (Qiagen) according to the manufacturer's protocol. After transfection, the transfected cells were selected using neomycin. After 5 passages, each Flag fusion protein was purified from HEK-293 cells by using the FLAG M Purification kit (Sigma) according to the manufacturer's protocol. We purified the protein from the HEK-293 cells transfected with the Tag4 vector containing the N-DDR1 coding region; this protein contained the collagen-binding site of DDR1 and was used as DDR1-blocking protein. In addition, we purified the protein from the THK-293 cells transfected with the Tag4 vector containing the C-DDR1 coding region; this protein does not include the collagen-binding site of DDR1 and was used as the control protein.
Apoptosis assay
Eosinophils were incubated in the presence or absence of an agonistic anti-CD95 antibody at a concentration of 1 × 106 cells (Fas agonistic antibody, UT-1, mouse IgM; Acris Antibodies, Hiddenhausen, Germany), mouse IgM (isotype control for the Fas agonistic antibody; Santa Cruz Biotechnology), 50 μg/mL type I collagen (Sigma-Aldrich, St Louis, MO), caffeic acid phenethyl ester (CAPE) (NF-κB inhibitor; Calbiochem, San Diego, CA), β1-blocking antibody (mouse IgG, DE9 10 μg/mL; Upstate Biotechnology, Lake Placid, NY), DDR1 agonistic antibodies, control IgM,10,12,14,15 and DDR1-blocking protein or control protein. After incubation under various conditions, 1 × 106 eosinophils were collected and exposure of phosphatidylserine was detected by flow cytometry analysis as described previously18 by using the annexin V–FITC antibody (PharMingen) and 7-amino-actinomycin D (7AAD; PharMingen). In all experiments, apoptotic data were confirmed by a TdT-mediated dUTP nick-end labeling (TUNEL) assay performed using a commercially available kit according to the manufacturer's instructions (TUNEL Label Mix; Roche Diagnostics, Basel, Switzerland).
Rhodamine-123 (Molecular Probes, Eugene, OR) was used to access changes in the mitochondrial membrane potential. In brief, the cells were incubated with 10 μg/mL rhodamine-123 at 37°C for 30 minutes. After incubation, the cells were washed with PBS and subsequently analyzed by flow cytometry analysis.
Western blot analysis
To detect the DDR1 isoforms, 1 × 107 eosinophils were lysed on ice for 20 minutes in 1 mL lysis buffer containing 50 mM HEPES, 150 mM NaCl, 1% Triton X-100, 10% glycerol, and a cocktail of protease inhibitors (Roche Diagnostics). The lysates were centrifuged, and 20 μL of the supernatant was collected. Subsequently, 20 μL of a double-strength sample buffer (20% glycerol, 6% SDS, and 10% 2-mercaptoethanol) was added to the supernatants. The samples were boiled for 10 minutes. The proteins were analyzed on 8% or 12% polyacrylamide gel by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically onto nitrocellulose membranes at 150 mA for 1 hour by using a semidry system. The membranes were incubated with rabbit IgGs specific for DDR1a,10 DDR1b,12 or both forms of the C-terminal of DDR1 (C-20; Santa Cruz Biotechnology) or with either of the following: the N-terminal of DDR1 (N-13; Santa Cruz Biotechnology), anti–human actin monoclonal mouse IgG antibodies (Santa Cruz Biotechnology), anti-Flag mouse IgG monoclonal antibody (Sigma), antiphosphorylated Bcl-2 rabbit IgG antibody, or anti–Bcl-2 rabbit IgG antibody (Cell Signaling Technology, Beverly, MA) followed by sheep anti–rabbit or anti–mouse IgGs coupled with horseradish peroxidase (Amersham, Arlington Heights, IL). The peroxidase activity was visualized using an enhanced chemiluminescence detection system (Amersham). The intensities of the DDR1 isoforms and actin were analyzed using the NIH Image Program (National Institutes of Health, Bethesda, MD), and the relative amount of each DDR1 isoform (DDR1 amount ratio) in each patient was then calculated.
To evaluate whether DDR1 activation by type I collagen or DDR1 agonistic antibodies induces autophosphorylation of DDR1 and DDR1 signal transduction, 1 × 107 eosinophils were plated on dishes, serum-starved in RPMI 1640 containing 1% FCS for 10 hours, and subsequently activated with 50 μg/mL type I collagen (Sigma) or DDR1 agonistic antibodies (513DDR1 antibody)10,12 ; the eosinophils were then cultured for 1 hour. Cell lysates were prepared, and DDR1 in the cell lysates was immunoprecipitated using anti-DDR1 antibodies (C-20; Santa Cruz Biotechnology) and recombinant protein G–agarose (Invitrogen, Gaithersburg, MD), as previously reported.10-15 Additionally, tyrosine phosphorylation of DDR1 and Shc recruitment were analyzed by Western blotting using mouse monoclonal antiphosphotyrosine IgGs (4G10; Upstate Biotechnology) or mouse monoclonal anti-Shc antibodies (R&D Systems, Minneapolis, MN), followed by sheep anti–mouse IgGs coupled with horseradish peroxidase (Amersham). The peroxidase activity was visualized using the enhanced chemiluminescence detection system (Amersham).
Immunohistochemistry
Biopsied tissues that were obtained from 3 CSS patients were examined by immunohistochemical staining for DDR1 by using a rabbit anti-DDR1 antibody (Santa Cruz Biotechnology) and were visualized by employing the DAB method as described previously.19 Sections 4 μm thick were mounted on poly-l-lysine–coated slides, dewaxed, and washed in Tris-buffered saline (pH 7.4) for 10 minutes. For optimal antigen retrieval, the sections were pressure cooked in 0.01 M citrate buffer (pH 6.0) for 90 seconds. Endogenous peroxidase activity was blocked using a 3% hydrogen peroxide solution in methanol for 10 minutes. Following 2 washes in PBS with 1% saponin, the blocking reaction was performed as reported previously.20 The sections were incubated with a primary antibody solution for 2 hours at room temperature by using a 1:50 dilution of the antibody. The negative control slides were incubated with rabbit IgG (R&D Systems). A secondary biotinylated anti-Ig antibody (R&D Systems) was added, and the mixture was incubated for 30 minutes at room temperature. After washing, the sections were incubated with streptavidin conjugated with horseradish peroxidase (Amersham) and then rinsed with deionized water. The DAB substrate solution was added, and the mixture was incubated for 10 minutes. A brown-colored reaction indicated a positive result.
ELISA assay
Eosinophils (1 × 106/mL) from CSS patients or asthma patients were incubated with DDR1 agonistic antibody10,12 or cultured on type I collagen– (Sigma) coated dish for 2 hours or 24 hours in the presence or absence of DDR1-blocking protein, control protein, or cycloheximide (Calbiochem), as applicable. Following culture, the supernatants were collected, and the concentrations of granulocyte-macrophage colony-stimulating factors (GM-CSFs) and interleukin-6 (IL-6) were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) according to the manufacturer's protocols.
EMSA
A total of 1 × 108 eosinophils were incubated with DDR1 agonistic antibody,10,12 control IgM, collagen, β1-blocking antibody (mouse IgG, DE9 10 μg/mL; Upstate Biotechnology), DDR1-blocking protein, and control protein (for 6 hours) and nuclear extracts were prepared as previously described10 ; aliquots were frozen at −80°C. For the experiments that used CAPE, a specific inhibitor of the nuclear transcription factor NF-κB, we incubated cells with CAPE (10 μg/mL; Calbiochem) for 30 minutes prior to the stimulation, as described above. For electrophoretic mobility shift assay (EMSA), end-labeled 32 P-oligonucleotide probes corresponding to the NF-κB–binding site of the Ig κ-chain gene (5′-AGTTGAGGGGACTTTCCCAGGC-3′) were incubated with 5 μg nuclear extracts in a 20 μL binding mixture (50 mM Tris-HCl [pH 7.4], 25 mM MgCl2, 0.5 mM DTT, and 50% glycerol) at 4°C for 15 minutes. The DNA-protein complexes were resolved on a 5% polyacrylamide gel. The gels were dried and then exposed to x-ray films.
RNA interference
A mixture of small interfering RNAs (siRNAs) specific for DDR1 was purchased from Santa Cruz Biotechnology. Further, we constructed mutant siRNAs for DDR1 as a control for DDR1 siRNA. The sequences of the mutated siRNA oligonucleotides were 5′-AAGTGTTTCTATTTACTGG-3′ and 3′-CCAGTAAATAGAAACACTT-5′ (underlining indicates the mutated oligonucleotides).
A total of 1 × 106 cells per milliliter of eosinophils in complete medium were transfected with siRNA at a final concentration of 100 nM by using an siRNA transfection reagent (Santa Cruz Biotechnology) according to the manufacturer's protocol. After 48 hours of incubation, the cells were rinsed with PBS and used for further analysis as described above.
Statistical analysis
We used the Bonferroni-Dunn test with 1-way factorial analysis of variance (ANOVA) and Wilcoxon signed rank test. A P value below .05 was considered significant. Values were presented as the mean ± standard deviation unless stated otherwise.
Results
DDR1 expression on peripheral eosinophils
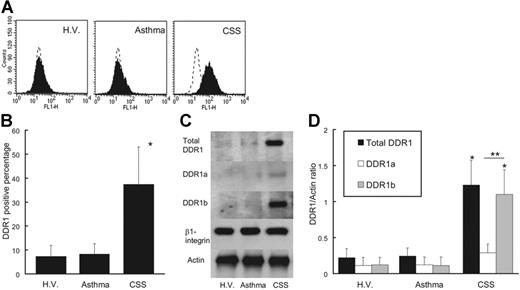
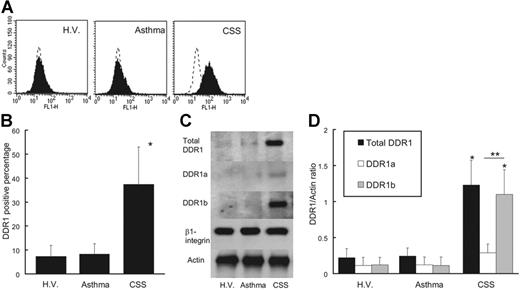
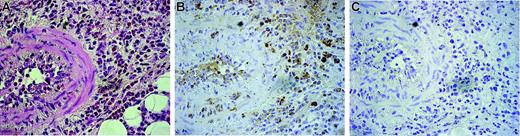
As shown in Figure 1, peripheral eosinophils from CSS patients expressed endogenous DDR1. The DDR1-positive percentage of CSS patients was significantly higher than that of asthma patients and healthy volunteers (Figure 1A-B). The total amount of DDR1 protein was also higher per 1 × 107 eosinophils in CSS patients, and the proportion of cells expressing the DDR1b isoform was also considerably higher in the eosinophils of these patients (Figure 1C-D). There was no significant difference in the concentrations of β1 integrin and another collagen receptor among the 3 groups. Immunohistochemical analysis revealed that the eosinophil staining in the blood vessels and in the wall of the blood vessels was positive for DDR1. Tissue-infiltrating eosinophils also stained positive for DDR1 (Figure 2).
DDR1 expression of eosinophils. (A) Flow cytometry analysis of DDR1 expression on peripheral blood eosinophils from CSS patients, asthma patients, and healthy volunteers (H.V.). (B) Comparison of DDR1 expression level among CSS patients, asthma patients, and healthy volunteers (*P < .001, Bonferroni-Dunn test with 1-way factorial ANOVA). (C) Western blot analysis of total DDR1, DDR1a, and DDR1b in peripheral blood eosinophils from CSS patients, asthma patients, and healthy volunteers. (D) Comparison of total DDR1 and DDR1 isoform amount among CSS patients, asthma patients, and healthy volunteers (*P < .001 when compared with asthma patients and healthy volunteers; **P < .01, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
DDR1 expression of eosinophils. (A) Flow cytometry analysis of DDR1 expression on peripheral blood eosinophils from CSS patients, asthma patients, and healthy volunteers (H.V.). (B) Comparison of DDR1 expression level among CSS patients, asthma patients, and healthy volunteers (*P < .001, Bonferroni-Dunn test with 1-way factorial ANOVA). (C) Western blot analysis of total DDR1, DDR1a, and DDR1b in peripheral blood eosinophils from CSS patients, asthma patients, and healthy volunteers. (D) Comparison of total DDR1 and DDR1 isoform amount among CSS patients, asthma patients, and healthy volunteers (*P < .001 when compared with asthma patients and healthy volunteers; **P < .01, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
Immunohistochemical analysis of DDR1 expression in the biopsied tissue of a CSS patient. Original magnification, × 350. (A) Hematoxylin-eosin staining. (B) Stained with the DDR1 antibody. (C) Negative control for the DDR1 antibody. Cells were stained with hematoxylin. Images were captured with an Olympus U-PMTVC microscope (Olympus, Tokyo, Japan) with a UPlan Apo 20×/0.80 NA objective lens, and an Olympus DP11-N. Images were captured with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Immunohistochemical analysis of DDR1 expression in the biopsied tissue of a CSS patient. Original magnification, × 350. (A) Hematoxylin-eosin staining. (B) Stained with the DDR1 antibody. (C) Negative control for the DDR1 antibody. Cells were stained with hematoxylin. Images were captured with an Olympus U-PMTVC microscope (Olympus, Tokyo, Japan) with a UPlan Apo 20×/0.80 NA objective lens, and an Olympus DP11-N. Images were captured with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Stimulation with collagen or DDR1 agonistic antibodies transducted DDR1 signaling and nuclear translocation of NF-κB in the eosinophils from the CSS patients
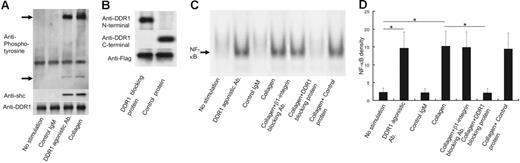
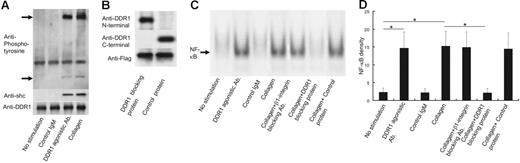
To evaluate whether collagen or DDR1 agonistic antibodies induce DDR1 activation and signal transduction in the eosinophils of CSS patients, we performed Western blotting against phosphorylated DDR1 and Shc—an adaptor protein for DDR1b signal transduction.13 As shown in Figure 3A, collagen or DDR1 agonistic antibodies induced DDR1 phosphorylation and Shc recruitment. This experiment was repeated 5 times by using the eosinophils from 5 CSS patients, and the same results were obtained for each experiment.
DDR1 activation and NF-κB activation in the eosinophils of CSS patients. (A) Western blot analysis for phospholyrated DDR1 and Shc by the stimulation with DDR1 agonistic antibody, control IgM, and collagen. (B) Western blot analysis for Flag fusion protein with or without collagen-binding site of DDR1. (C) Analysis of nuclear NF-κB translocation in the eosinophils of CSS patients by the stimulation with DDR1 agonistic antibody, control IgM, and collagen in the presence or absence of β1 integrin–neutralizing antibody, DDR1-blocking protein, and control protein. (D) Comparison of NF-κB density (*P < .001, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
DDR1 activation and NF-κB activation in the eosinophils of CSS patients. (A) Western blot analysis for phospholyrated DDR1 and Shc by the stimulation with DDR1 agonistic antibody, control IgM, and collagen. (B) Western blot analysis for Flag fusion protein with or without collagen-binding site of DDR1. (C) Analysis of nuclear NF-κB translocation in the eosinophils of CSS patients by the stimulation with DDR1 agonistic antibody, control IgM, and collagen in the presence or absence of β1 integrin–neutralizing antibody, DDR1-blocking protein, and control protein. (D) Comparison of NF-κB density (*P < .001, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
To confirm whether eosinophil activation by collagen is dependent on DDR1, we constructed Flag fusion proteins with and without the collagen-binding site of DDR1. As shown in Figure 3B, the Flag fusion protein that includes the collagen-binding site of DDR1 (so-called DDR1-blocking protein) stained positive for the anti-DDR1 N-terminal antibody but not for the anti-DDR1 C-terminal antibody. On the other hand, the Flag fusion protein without the collagen-binding site of DDR1 (the so-called control protein) stained positive for the anti-DDR1 C-terminal antibody but not for the anti-DDR1 N-terminal antibody. Both proteins stained positive for the anti-Flag antibody. We stimulated eosinophils from CSS patients with collagen in the presence of these proteins and examined phosphorylated DDR1. Addition of 1 mg/mL DDR1-blocking protein significantly attenuated collagen-induced DDR1 phosphorylation, while the same amount of control protein did not inhibit the collagen-induced DDR1 phosphorylation (data not shown). Therefore, we used these proteins to confirm whether eosinophil activation by collagen is dependent on DDR1.
DDR1 stimulation can induce NF-κB activation.10,11,15 Therefore, we examined the nuclear translocation of NF-κB in the eosinophils. As shown in Figure 3C-D, collagen or DDR1 agonistic antibodies induced the nuclear translocation of NF-κB in the eosinophils of the CSS patients. Control IgM did not induce the nuclear translocation of NF-κB. The neutralizing antibodies of β1 integrin—another collagen receptor—did not affect the nuclear translocation of NF-κB induced by collagen. The addition of the DDR1-blocking protein (1 mg/mL) significantly attenuated eosinophil NF-κB activation induced by collagen. Addition of the control protein (1 mg/mL) did not affect eosinophil NF-κB activation induced by collagen. The attenuation effect of the DDR1-blocking protein was observed at a concentration of 0.3 mg/mL and reached the peak at 1 mg/mL and a plateau (data not shown). This experiment was repeated 5 times by using the eosinophils from 5 CSS patients, and the same results were obtained from each experiment (Figure 3C).
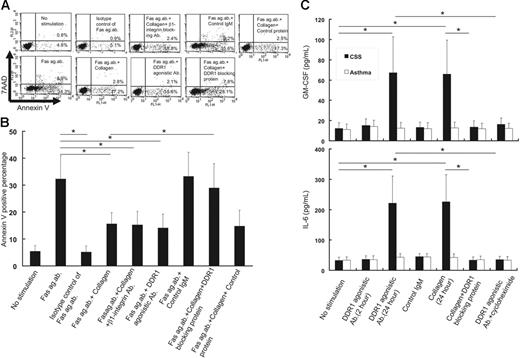
Stimulation with collagen or DDR1 agonistic antibodies prevented CD95 agonistic antibody–induced apoptosis of eosinophils from CSS patients
In a preliminary study, we cultured eosinophils from CSS patients in various concentrations of agonistic anti-CD95 antibody (1, 5, 10, 50, 100, and 500 μg/mL) and found that 100 μg/mL is the optimal concentration to induce apoptosis. The apoptosis rate was highest at a concentration of 100 μg/mL and then showed a peak (data not shown). As shown in Figure 4, addition of 100 μg/mL Fas agonistic antibody induced apoptosis of eosinophils; however, the addition of the same concentration of a Fas agonistic antibody isotype control (100 μg/mL, mouse IgM) did not induce apoptosis. Collagen or DDR1 agonistic antibodies significantly inhibited the agonistic anti-CD95 antibody–induced apoptosis. The inhibitory effect of the DDR1 agonistic antibody was higher at a concentration of 1 μg/mL than at a concentration of 0.1 μg/mL. The control IgM (1 μg/mL) did not exert any effect on the agonistic anti-CD95 antibody–induced apoptosis. The neutralizing antibodies of β1 integrin, another collagen receptor,21 did not affect the antiapoptotic effect of collagen. Addition of the DDR1-blocking protein (1 mg/mL) significantly attenuated the antiapoptotic effect of collagen, while the control protein (1 mg/mL) did not exert any affect.
Functional analysis of DDR1 stimulation on eosinophils from CSS patients. (A) Flow cytometry analysis of apoptotic cells by the stimulation with Fas agonistic antibody in the presence or absence of DDR1 agonistic antibody, control IgM, β1 integrin–neutralizing antibody, DDR1-blocking protein, and control protein. The x-axis indicates annexin V–positive cells, and the y-axis indicates 7AAD-positive cells. (B) Comparison of annexin V–positive cells in various conditions (*P < .01, Bonferroni-Dunn test with 1-way factorial ANOVA). (C) Comparison of GM-CSF and IL-6 production from the eosinophils of CSS patients and asthma patients in various conditions (*P < .001 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
Functional analysis of DDR1 stimulation on eosinophils from CSS patients. (A) Flow cytometry analysis of apoptotic cells by the stimulation with Fas agonistic antibody in the presence or absence of DDR1 agonistic antibody, control IgM, β1 integrin–neutralizing antibody, DDR1-blocking protein, and control protein. The x-axis indicates annexin V–positive cells, and the y-axis indicates 7AAD-positive cells. (B) Comparison of annexin V–positive cells in various conditions (*P < .01, Bonferroni-Dunn test with 1-way factorial ANOVA). (C) Comparison of GM-CSF and IL-6 production from the eosinophils of CSS patients and asthma patients in various conditions (*P < .001 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
Stimulation with a DDR1 agonistic antibody or collagen induced cytokine productions from eosinophils
Because NF-κB was reported to be involved in the production of several cytokines in eosinophils,22,23 we examined cytokine concentrations in the supernatants of eosinophils stimulated with the DDR1 agonistic antibody or collagen that was coated on the dishes. As shown in Figure 4C, incubation with the DDR1 agonistic antibody or collagen for 24 hours induced GM-CSF and IL-6 production from the eosinophils of CSS patients. However, incubation with DDR1 agonistic antibody for 2 hours did not induce cytokine production. Incubation with collagen for 2 hours did not induce cytokine production (data not shown). Addition of the DDR1-blocking protein (1 mg/mL) significantly attenuated collagen-induced cytokine production, while that with the control protein (1 mg/mL) did not. This induction of cytokine production by collagen was not inhibited by the neutralizing antibody of β1 integrin, another collagen receptor (data not shown).
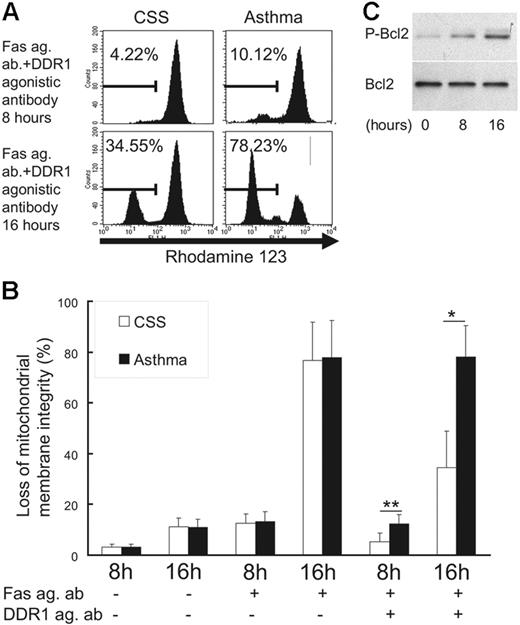
DDR1 activation reduced loss of mitochondrial membrane integrity and induced Bcl-2 phosphorylation
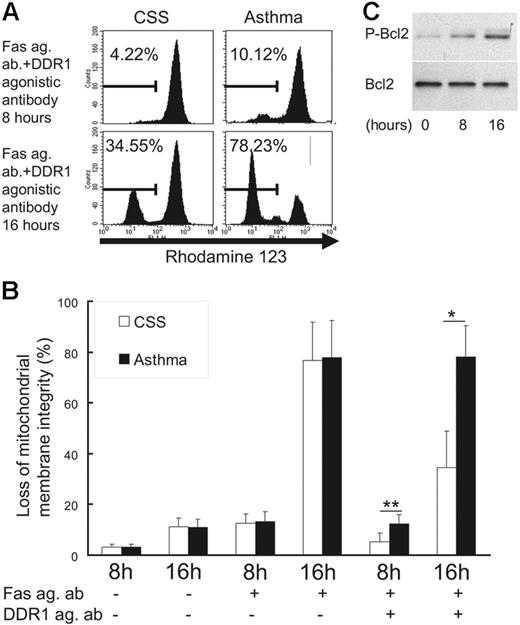
To investigate the molecular mechanism underlying the inhibition of Fas-induced apoptosis by DDR1 crosslinking, we examined mitochondrial integrity and phosphorylation of Bcl-2, a compound with the ability to exert a survival function in response to a wide range of apoptotic stimuli by blocking the mitochondrial release of cytochrome c.24 As shown in Figure 5, when the eosinophils were incubated with the Fas agonistic antibody in the presence of the DDR1 agonistic antibody, the loss of mitochondrial membrane integrity was significantly lower in CSS patients than in asthma patients (Figure 5A-B). Also, incubation with a DDR1 agonistic antibody induced Bcl-2 phosphorylation in eosinophils of CSS patients (Figure 5C).
Change of mitochondrial integrity and Bcl-2 phosphorylation in eosinophils. (A) Flow cytometry analysis for rhodamine-123 in eosinophils. (B) Comparison of the loss of mitochondrial membrane integrity between CSS patients and asthma patients in various conditions (Fas ag ab indicates Fas agonistic antibody; DDR1 ag ab, DDR1 agonistic antibody; *P < .01, **P < .05, Bonferroni-Dunn test with 1-way factorial ANOVA). (C) Western blot analysis of Bcl-2 and phosphorylated Bcl-2 in eosinophils from CSS patients in the presence of DDR1 agonistic antibody. Error bars indicate standard deviation.
Change of mitochondrial integrity and Bcl-2 phosphorylation in eosinophils. (A) Flow cytometry analysis for rhodamine-123 in eosinophils. (B) Comparison of the loss of mitochondrial membrane integrity between CSS patients and asthma patients in various conditions (Fas ag ab indicates Fas agonistic antibody; DDR1 ag ab, DDR1 agonistic antibody; *P < .01, **P < .05, Bonferroni-Dunn test with 1-way factorial ANOVA). (C) Western blot analysis of Bcl-2 and phosphorylated Bcl-2 in eosinophils from CSS patients in the presence of DDR1 agonistic antibody. Error bars indicate standard deviation.
Suppression of DDR1 expression in CSS eosinophils attenuated the antiapoptotic effect and cytokine production of collagen or DDR1 agonistic antibodies
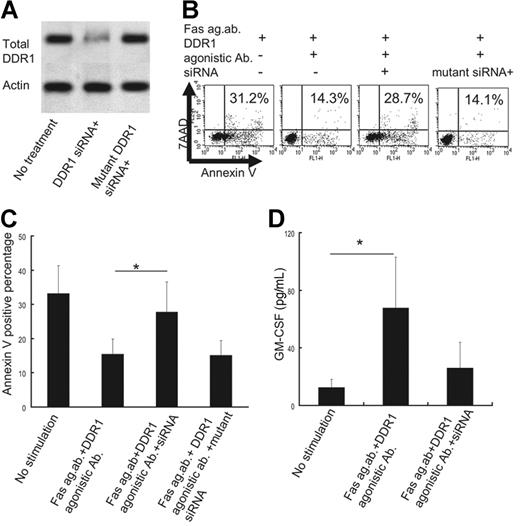
DDR1 siRNA (Santa Cruz Biotechnology) significantly inhibited endogenous DDR1 expression in the eosinophils obtained from the CSS patients; this was not observed in the case of the mutated DDR1 siRNA (Figure 6A). The suppression of DDR1 expression by siRNA significantly attenuated the antiapoptotic effect of collagen or DDR1 agonistic antibodies against eosinophils from CSS patients, while the mutated DDR1 siRNA did not exert any such effects (Figure 6B-C). Also, the suppression of DDR1 expression by siRNA significantly decreased GM-CSF production from the eosinophils of CSS patients, while the mutated DDR1 siRNA did not (Figure 6D).
Effect of DDR1 suppression by siRNA. (A) Western blot analysis for DDR1 and actin in the presence or absence of DDR1 siRNA or mutated DDR1 siRNA. (B) Flow cytometry analysis of apoptotic cells in the presence or absence of DDR1 siRNA or mutated DDR1 siRNA. The x-axis indicates annexin V–positive cells, and the y-axis indicates 7AAD-positive cells. (C) Comparison of annexin V–positive cells in the presence or absence of DDR1 siRNA or mutated DDR1 siRNA *P < .01 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). (D) Comparison of GM-CSF production from eosinophils of CSS patients in the presence or absence of DDR1 siRNA (**P < .01 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
Effect of DDR1 suppression by siRNA. (A) Western blot analysis for DDR1 and actin in the presence or absence of DDR1 siRNA or mutated DDR1 siRNA. (B) Flow cytometry analysis of apoptotic cells in the presence or absence of DDR1 siRNA or mutated DDR1 siRNA. The x-axis indicates annexin V–positive cells, and the y-axis indicates 7AAD-positive cells. (C) Comparison of annexin V–positive cells in the presence or absence of DDR1 siRNA or mutated DDR1 siRNA *P < .01 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). (D) Comparison of GM-CSF production from eosinophils of CSS patients in the presence or absence of DDR1 siRNA (**P < .01 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
An NF-κB inhibitor attenuated the antiapoptotic effect of collagen or DDR1 agonistic antibodies
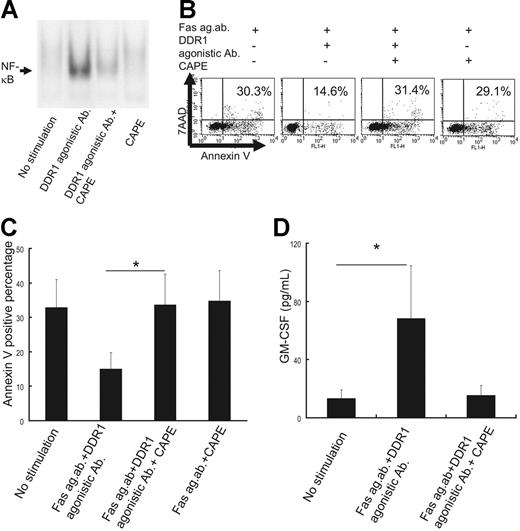
To evaluate whether NF-κB contributes to the antiapoptotic effect and cytokine production on stimulation with collagen or DDR1 agonistic antibodies, we cultured eosinophils with CAPE, an NF-κB–specific inhibitor. In a preliminary study, we cultured eosinophils in various concentrations of CAPE (0.1, 1, 5, 10, 50, and 100 μg/mL) and found that 10 μg/mL is the optimal concentration for suppressing NF-κB translocation (data not shown). As shown in Figure 7, CAPE significantly inhibited DDR1-induced NF-κB activation (Figure 7A) and attenuated the antiapoptotic effect of collagen or DDR1 agonistic antibodies (Figure 7B-C). Independently, however, CAPE did not affect the Fas agonistic antibody–induced eosinophil apoptosis (Figure 7B-C). Additionally, CAPE significantly decreased DDR1-induced GM-CSF production from the eosinophils of CSS patients (Figure 7D).
Effect of NF-κB–specific inhibitor CAPE. (A) Analysis of nuclear NF-κB translocation in the eosinophils of CSS patients in the presence or absence of CAPE. (B) Flow cytometry analysis of apoptotic cells in the presence or absence of CAPE. (C) Comparison of annexin V–positive cells in the presence or absence of CAPE. *P < .01 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). (D) Comparison of GM-CSF production from eosinophils of CSS patients in the presence or absence of CAPE (*P < .01 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
Effect of NF-κB–specific inhibitor CAPE. (A) Analysis of nuclear NF-κB translocation in the eosinophils of CSS patients in the presence or absence of CAPE. (B) Flow cytometry analysis of apoptotic cells in the presence or absence of CAPE. (C) Comparison of annexin V–positive cells in the presence or absence of CAPE. *P < .01 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). (D) Comparison of GM-CSF production from eosinophils of CSS patients in the presence or absence of CAPE (*P < .01 as compared with no stimulation, Bonferroni-Dunn test with 1-way factorial ANOVA). Error bars indicate standard deviation.
Discussion
This is the first study reporting the demonstration of the antiapoptotic effect of DDR1 on the eosinophils of the CSS patients. Prominent eosinophilia is one of the defining features of CSS.3 Its magnitude commonly reflects clinical disease activity and, in many situations, eosinophil suppression results in clinical improvement.3,25,26 CSS can virtually affect any organ system in the body27,28 ; therefore, systemic symptoms are prominent in CSS.1,5 Analysis of tissue biopsy specimens from patients with CSS shows an eosinophil-rich inflammatory infiltrate with granuloma formation in the connective tissue and blood vessel walls.1,5 Eosinophils can cause tissue injury by releasing a spectrum of toxic products such as the eosinophil cationic protein.1,5,29 Thus, eosinophils play an important role in the pathogenesis of CSS; therefore, the elucidation of the survival mechanism of eosinophils in the tissue microenvironment is necessary. In this regard, fibronectin—a component of the ECM—and adhering molecules such as intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 can prolong eosinophil survival via interaction with their receptor, integrins.30-34 In primary systemic vasculitis, including CSS, increased levels of the fibronectin-containing complex have been reportedly observed in the serum.35 Thus, the interaction between the eosinophil surface molecules and the components in the tissue microenvironment appears to play a pivotal role in eosinophil survival. Migration of eosinophils into specific tissue sites is the hallmark of CSS.1,5 In this process, eosinophils must establish contact with the basement membrane, which is abundant in the DDR1 ligand, namely, collagen.36 In our study, DDR1 stimulation induced NF-κB activation, and NF-κB has been reported to play a pivotal role in the prevention of apoptosis.37 Therefore, we believe that the interaction between endogenous DDR1 on eosinophils and collagen in the ECM might affect eosinophil survival in the tissue microenvironment in CSS. In our study, the neutralizing antibody of β1 integrin, another collagen receptor, did not influence the antiapoptotic effect of collagen. In fact, the DDR1-blocking protein attenuated the collagen-induced antiapoptotic effect. Collagen also binds to non-DDR1 collagen receptors such as β2 integrin. Although the collagen–β2 integrin interaction enhances platelet-activating factor–induced eosinophil chemotaxis,38,39 there has been no report suggesting the antiapoptotic effect of β2 integrin thus far. Of course, we cannot deny the possible contribution of collagen–β2 integrin interaction to the collagen-induced antiapoptotic effect from this study. We think that the antiapoptotic effect of collagen-DDR1 interaction might be one of the molecular interactions that contribute to eosinophil survival in the tissue microenvironment in conjunction/combination with other mechanisms.
In this study, DDR1 activation on eosinophils induced the production of several cytokines in an NF-κB–dependent manner. Previously, we found that DDR1 could induce NF-κB activation in leukocytes and pulmonary fibroblasts and that it contributed to cytokine production and had an antiapoptotic effect.10,11,15 In eosinophils, NF-κB was reported to be associated with the production of cytokines such as GM-CSF22 and IL-6.23,40 In primary systemic vasculitis, including CSS, the Th2 cytokine cascade, including IL-6, was reported to be activated.29,41-43 Particularly, autocrine production of GM-CSF by stimulation with interleukin-15 was reported to be associated with eosinophil survival in an NF-κB–dependent manner.44 Thus, NF-κB activation appears to play an important role in maintaining eosinophil functions and in the pathogenesis of CSS. In our study, DDR1 activation on eosinophils from CSS patients induced GM-CSF production in an NF-κB–dependent manner. Our study also showed that DDR1-induced cytokine production required de novo protein synthesis and that DDR1 activation did not induce the release of stored IL-6 and GM-CSF. DDR1 might be one of the upstream signaling molecules that can activate NF-κB of eosinophils in CSS. GM-CSF production by stimulation with noncollagen ECM components—laminin and fibronectin—contributes to eosinophil survival.34 The DDR1 expression level in the eosinophils of CSS patients was significantly higher than that in the eosinophils of asthma patients. DDR1 activation could induce GM-CSF production in the eosinophils of CSS patients. Taken together, we believe that DDR1 activation might induce autocrine GM-CSF production from eosinophils that contributes to eosinophil survival in the tissue microenvironment of CSS.
This is the first study that demonstrates functional DDR1 expression on eosinophils. It was reported that 2 DDR1 isoforms—DDR1a and DDR1b—could be induced in human neutrophils, monocytes, and lymphocytes.10,12,13,16 Overexpression of DDR1a in the human monocytic leukemic cell line THP-1 promoted their migration through 3-dimensional collagen lattices.13 Further, collagen activation of DDR1b promoted PMA-induced differentiation of THP-1 cells, dendritic cell maturation, and cytokine production from human macrophages.10,12 Disruption of the DDR1 gene in mice resulted in viable animals that were significantly smaller than their littermates, and female DDR1-null mice showed defects in blastocyst implantation and mammary gland development.45 Primary vascular smooth muscle cells that were isolated from the DDR1-null mice showed decreased proliferation, collagen attachment, and migration in vitro.46,47 In contrast, primary mesangial cells isolated from the kidney of the DDR1-null mice showed enhanced proliferation.48 These previous observations have indicated that DDR1 plays a role in cell attachment, migration, and proliferation. Eosinophils express numerous surface molecules that are associated with cell adhesion, migration, and activation; these molecules are involved in the pathogenesis of the diseases with eosinophilia.49,50 DDR1 may be one of these molecules. Further studies that address DDR1 in eosinophils might provide a novel insight in clarifying the pathogenesis of diseases with eosinophilia.
In our study, we found that DDR1 activation induced the phosphorylation of Bcl-2 in eosinophils from CSS patients. Bcl-2 can exert a survival function in response to a wide range of apoptotic stimuli by blocking the mitochondrial release of cytochrome c.24 Recent evidence indicates that the antiapoptotic functions of Bcl-2 can be regulated by posttranslational modifications, including phosphorylation.51 Growth factors such as interleukin-3 and JNK can induce Bcl-2 phosphorylation, which is necessary for enhanced antiapoptotic effect52 ; however, certain aspects of the signaling pathway remain controversial. On the other hand, downstream of DDR1 signaling has not been fully elucidated. DDR1 has a total of 15 tyrosine residues in its cytoplasmic regions, which serve as potential phosphorylation sites on receptor activation by collagen.53 Sustained phosphorylation of DDR1 will potentially facilitate binding of a number of different Src homology-2 (SH2) domain– and phosphotyrosine-binding (PTB) domain–containing molecules. Additionally, downstream events may be highly cell-type dependent.54 For example, in macrophages, DDR1-induced Shc phosphorylation led to the activation of the TRAF6 complex, triggering the p38 mitogen-activated protein kinase and NF-κB pathways.10-15 However, in human breast cancer T-47D cells, it results in strong Shc binding to the receptor but not in protein phosphorylation.7,8 Our study provided a novel insight on these aspects for the investigation of Bcl-2 and DDR1 signaling pathways.
Previous reports on the effect of collagen on eosinophil survival are inconsistent. Tourkin et al55 reported that collagen could prolong eosinophil survival. However, Walsh and Wardlaw34 reported that the effect of collagen on eosinophil survival was lower than that of laminin or fibronectin, and they insisted that approximately 9% of mononuclear cell contamination might be responsible for the effects of collagen on eosinophil survival. In our study, we used the microbead technique for selecting eosinophils of high purity.56 In fact, the eosinophil percentage was more than 99% in our study. The expression level of DDR1 on eosinophils was significantly higher in CSS patients than in asthma patients, and collagen could inhibit the Fas (CD95) agonistic antibody–induced eosinophil apoptosis only in CSS patients. Suppression of DDR1 expression by siRNA significantly reduced the antiapoptotic effect of DDR1 activation. Therefore, we believe that collagen-DDR1 interaction contributes to eosinophil survival in the tissue microenvironment at least in CSS. The sample size used in our study was too small to draw a definitive conclusion; therefore, we propose the necessity for further studies addressing this point and thereby clarifying the DDR1-collagen effect on eosinophil in CSS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
This work was supported by a grant-in-aid for scientific research (no. 18790542) from the Japan Society for the Promotion of Science (JSPS) and grants from the Sumitomo Foundation (no. 040010), Nagao Memorial Fund, Uehara Memorial Foundation, and Kanae Foundation for Life and Socio-Medical Science. We offer special thanks to Mrs Rumi Matsuyama (Third Department of Internal Medicine, Kagoshima University Hospital) for her excellent help.