Abstract
In HLA-incompatible hematopoietic stem cell transplantation, alloreactive donor T cells recognizing recipient mismatch HLA cause severe graft-versus-host disease (GVHD). Strategies allowing the selective depletion of alloreactive T cells as well as the enhancement of graft-versus-malignancy immunity would be beneficial. We generated donor CD8 T-cell lines in vitro using allogeneic recipient cells mismatched at a single HLA class I allele or haplotype as stimulators. Recipient cells were obtained from acute myeloid leukemias, renal-cell carcinomas, and CD40L-induced B lymphoblasts. Resulting alloreactive T cells were activated by incubating day 21 T-cell cultures with HLA-mismatch transfected K562 cells or recipient-derived fibroblasts. Selective allodepletion (SAD) was subsequently performed by a newly developed immunomagnetic depletion approach targeting the tumor necrosis factor receptor molecule CD137 (4-1BB). Compared with other activation-induced antigens, CD137 showed a superior performance based on a consistently low baseline expression and a rapid up-regulation following alloantigen stimulation. In 15 different SAD experiments, the frequency of alloreactive CD8 T cells was reduced to a median of 9.5% compared with undepleted control populations. The allodepleted T-cell subsets maintained significant antitumor and antiviral CD8 responses. In vitro expansion of tumor-reactive T cells followed by CD137-mediated SAD might enhance the antitumor efficacy of T-cell allografts with lower risk of inducing GVHD.
Introduction
For many patients with leukemia lacking a matched hematopoietic stem cell donor, transplantation of HLA-incompatible stem cells remains the only curative treatment option.1 However, donor-patient disparities at single or multiple HLA alleles increase the risks of graft rejection or severe graft-versus-host disease (GVHD).2,4-7 Both complications are mainly mediated by alloreactive T cells that recognize oligopeptides in association with foreign HLA molecules.8,9 T-cell depletion (TCD) is an efficient means for reducing alloreactivity10 and is mandatory if donors share only one HLA haplotype with the recipients.11 In this haploidentical setting, however, rigorous TCD abrogates specific immunity against pathogens and leukemia cells resulting in higher mortality from infections and disease relapse.12,13
The precursor frequency of alloreactive T cells is much higher than that of T cells recognizing infectious agents or malignant cells.14-16 Thus, depletion strategies need to substantially reduce alloreactive T cells, while sparing antipathogen and antitumor specificities. Various selective allodepletion (SAD) approaches have been developed for this purpose. Most of them rely on the ex vivo stimulation of donor lymphocytes derived from peripheral blood or bone marrow samples against recipient alloantigen-presenting cells over 1 to 7 days. Activated alloreactive T cells are subsequently depleted either by concomitant costimulatory blockade17 ; by photodynamic purging18,19 ; by fluorescence-activated cell sorting20,21 ; by CD95-mediated apoptosis22 ; or by targeting the activation-induced antigens CD25,23-28 CD69,29-31 CD71, and HLA-DR.24 Two of these approaches already demonstrated in vivo efficacy in terms of GVHD reduction and graft survival in haploidentical transplantation.17,32,33 However, disease relapse remained the major complication in patients receiving allodepleted grafts. This might be explained by the inadequately low precursor frequencies of leukemia-reactive T cells within infused lymphocytes.
We describe herein an alternative approach that is based on the in vitro expansion of donor T-cell lines through repeated stimulations with allogeneic leukemia or tumor cells prior to SAD. Because responding T cells durably expressed high levels of CD25, CD69, and CD95, we searched for alternative activation-induced antigens suitable for the allodepletion step. Among several candidates tested, the tumor necrosis factor receptor superfamily member CD137 (4-1BB)34,35 demonstrated the most favorable expression kinetic in allostimulated T-cell cultures. Using immunomagnetic CD137 depletion reagents we obtained a 1-log reduction of anti–HLA mismatch specificities in T-cell lines stimulated with HLA-incompatible leukemia or tumor cells. Allodepleted cultures showed considerable residual antitumor and antiviral CD8 T-cell responses. We also introduce HLA-negative K562 cells36 transfected with single HLA-class I molecules as an efficient tool to detect and deplete alloreactive T cells specifically for individual HLA alleles.
Materials and methods
Donors and cell lines
The study protocol was approved by the local Ethics Committee of the Landesaerztekammer Rheinland-Pfalz. Donors and recipients were healthy volunteers who participated in this study after providing informed consent in accordance with the Declaration of Helsinki. High-resolution HLA typing was performed from genomic DNA of donor and recipient cells (Visible Genetics, Cambridge, United Kingdom). HLA-class I genotypes are summarized in Table 1.
The renal-cell carcinoma (RCC) cell lines MZ1257-RCC and BA85-RCC were recently described.37 Acute myeloid leukemia (AML) blasts were harvested from peripheral blood of patients with leukemia with white blood cell counts exceeding 1011 /L. Blasts were isolated by Ficoll density centrifugation and were stored in liquid nitrogen until use. The allo-HLA-Cw*0702 reactive cytotoxic T lymphocyte (CTL) clone E77 was derived from a mixed-lymphocyte tumor cell culture (MLTC) sensitizing healthy donor CD8 T cells against HLA-C disparate RCC cells.37 HLA-negative K562 cells were transfected with full-length cDNAs encoding single HLA class I alleles according to a previously established protocol.38 Transfectants were selected in culture medium containing 1 mg/mL G418 (Gibco-BRL, Grand Island, NY) and were subsequently cloned. Only K562 clones with strong HLA surface expression were used as antigen-presenting cells (APCs). Culture medium was RPMI-1640 supplemented with L-glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% FCS (Cambrex Bio Science, Verviers, Belgium) (referred to as medium Ma).
Primary fibroblasts and B-cell blasts
Dermal fibroblast cultures were generated from 6-mm skin samples of patients undergoing plastic surgery. Briefly, the dermis was separated from epidermis after overnight incubation at 4°C in 2.4 U/mL Dispase II (Roche, Mannheim, Germany). Dermis was cultivated at 37°C in Dulbecco Modified Eagle Medium (Gibco-BRL) supplemented as described for medium Ma. Outgrowth of primary fibroblasts was usually observed after 3 to 4 weeks.
Peripheral-blood B cells were stimulated using NIH-3T3 cells stably transfected with human CD40 ligand (CD40L).39 CD40L cells were irradiated (100 Gy) and seeded in 6-well plates (Greiner, Nürtingen, Germany) at 4 × 105 cells/mL in Dulbecco Modified Eagle Medium/F-12 Nutrient (Gibco BRL) mixture (50:50, vol/vol) supplemented as described for medium Ma. After 18 to 24 hours, CD40L cells were cocultured with 2 × 106/mL peripheral-blood mononuclear cells (PBMCs) in Iscoves Modified Dulbecco Medium (Gibco-BRL) supplemented with 10% human serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 100 U/mL IL-4 (ImmunoTools, Friesoythe, Germany), 5 μg/mL insulin, and 500 ng/mL cyclosporine A (Sigma, St Louis, MO). Cultures were replated onto fresh irradiated CD40L cells in the presence of cyclosporine A and IL-4 every 3 to 4 days. The number of B cells was monitored weekly using a CD19 monoclonal antibody (mAb) (Beckman Coulter, Miami, FL) and flow cytometry. Usually, B-cell purity exceeded 90% after 2 to 3 weeks.
Allogeneic T-cell cultures
T-cell cultures were performed in 24-well plates (Greiner) in AIM-V medium (Gibco-BRL) supplemented with 5% human serum (referred to as medium Mb). Responders were pure CD4 or CD8 T-cell subpopulations (106/well) isolated from buffy coat PBMCs using CD4/CD8 Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). They were weekly stimulated with allogeneic irradiated (100 Gy) AML or RCC cells (each 105/well) in mixed lymphocyte-leukemia culture (MLLC) or mixed lymphocyte-tumor culture (MLTC), respectively. For mixed lymphocyte culture (MLC), purified CD8 buffy coat lymphocytes (106/well) were weekly stimulated with allogeneic irradiated (100 Gy) CD40L-induced B lymphoblasts (105/well). T-cell cultures were expanded by adding interleukin-2 (IL-2; Chiron, Emeryville, CA) on days 3 and 7 at 150 IU/mL. This concentration was reduced to 50 IU/mL on days 14, 21, and 28, which allowed sustained responder proliferation. From day 3 on throughout the whole culture period, medium was supplemented with 5 ng/mL IL-7 (R&D Systems, Wiesbaden, Germany).
CD137-mediated depletion of alloreactive T cells
On day 21 allogeneic T-cell cultures (106 cells/well) were stimulated once with irradiated (100 Gy) K562-HLA transfectant cells or recipient fibroblasts (105/well) in 2 mL medium Mb supplemented with 50 IU/mL IL-2 and 5 ng/mL IL-7. To increase HLA class I expression, fibroblasts were pretreated with 500 IU/mL IFN-γ (PromoCell, Heidelberg, Germany) over 3 days prior to use. After 24-hour incubation at 37°C, T cells were stained with a biotinylated CD137 mAb (clone 4B4-1; Axxora, San Diego, CA) alone or in combination with a biotinylated CD65 mAb (Dianova, Hamburg, Germany). In some experiments, biotinylated CD134 mAb (clone Ber Act 35; Axxora) instead of the anti-CD137 reagent was used. Labeling was performed at 6°C to 12°C over 15 minutes using 1 μL (10 μg) of each Ab per 107 responder cells in 80 μL AIM-V medium supplemented with 10 μg/mL DNAse (Roche). Following further 15 minutes of incubation with anti-Biotin microbeads (20 μL/107 cells in 80 μL AIM-V/DNAse) at 6°C to 12°C, the cell suspension was passed through an LD depletion column (Miltenyi Biotec). The column effluent, including 2 consecutive washings with 1 mL AIM-V/DNAse, was combined and designated as the CD137neg (or CD137/CD65neg) subset. For isolation of the CD137pos (or CD137/CD65pos) fraction, the column was removed from the separator, and cells were flushed out with 3 mL AIM-V/DNAse.
Flow cytometry analysis
Cells were incubated for 15 minutes at 4°C with fluoresceinisothiocyanate (FITC)– or phycoerythrin (PE)–conjugated mAbs. Abs were from Immunotech, Marseille, France, except for anti–CD134-PE, anti-CD137 (clone 4B4-1)–PE (both BD Biosciences, San Jose, CA), and the anti–HLA-B/-C mAb B1.23.2.40 For flow cytometry analysis, 104 events were collected after gating of viable lymphocytes by FSC and SSC signal list mode data in CellQuest Pro software on a fluorescence-activated cell sorting (FACS)–Calibur instrument (BD Biosciences).
Tetramer assay
HLA tetramers used to quantitate cytomegalovirus (CMV)–specific CD8 T cells within fresh PBMCs or T-cell bulk cultures included HLA-A*0201 folded with CMVpp65 peptide 495 to 503 NLVPMVATV41 and HLA-B*0702 folded with CMVpp65 peptide 417 to 426 TPRVTGGGAM42 (Beckman Coulter). For tetramer staining of Epstein-Barr virus (EBV)–specific CD8 T cells, HLA-A*0201 folded with EBV-BMLF1 peptide 280 to 288 GLCTLVAML43 was used (Beckman Coulter). Briefly, 5 × 105 cells were stained with 10 μL anti–CD8-PC5 mAb and 10 μL HLA tetramer-PE for 30 minutes at 12°C, washed once with 3 mL PBS, and resuspended in 500 μL 0.1% formaldehyde in PBS. To analyze the expression of activation molecules on CMV-specific T cells, samples were first incubated with FITC-labeled anti-CD69, anti-CD137, or IgG isotype control mAbs for 15 minutes at 12°C, washed with PBS, followed by tetramer staining to avoid activation through T-cell receptor (TCR) triggering. Stained cells were analyzed on FACS-Calibur and FACS-Canto (BD Biosciences) flow cytometers.
IFN-γ ELISPOT assay
IFN-γ enzyme-linked immunospot (ELISPOT) assays were performed as recently described.37 Briefly, T cells at 0.5 to 2 × 104/well and APCs at 1 to 5 × 104/well were seeded in ELISPOT plates in serum-free AIM-V medium. T cells without APCs served as controls. CD40L-induced B lymphoblasts and fibroblasts were pretreated with 500 IU/mL IFN-γ (over 3 days before use as APCs). After 20 hours of incubation at 37°C, IFN-γ spots were visualized and counted using an Axioplan 2 microscope combined with the computer-assisted image analysis system KS ELISpot 4.1 (Carl Zeiss Vision, Hallbergmoos, Germany).44 Results represent means of duplicate or triplicate wells.
51Cr release assay
Target cells were incubated for 90 minutes with 100 μCi (3.7 MBq) Na
Results
CD4 and CD8 T cells transiently express CD137 on antigen-specific stimulation
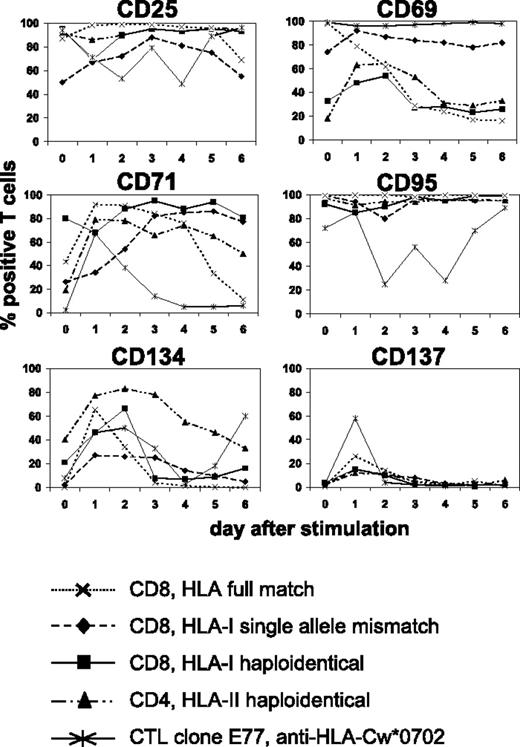
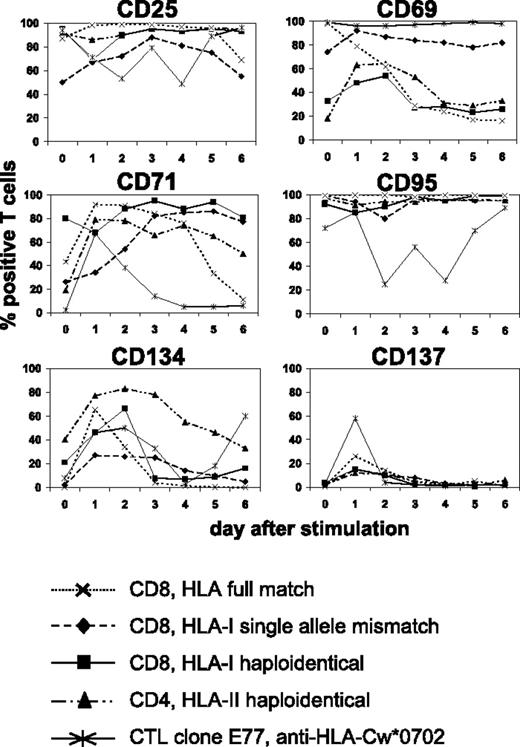
We searched for suitable cell-surface molecules allowing the selective depletion of alloreactive T cells from mixed lymphocyte-leukemia culture (MLLC) or mixed lymphocyte-tumor culture (MLTC), respectively. MLLC/MLTC responders were generated by 4 weekly in vitro stimulations of donor CD4 or CD8 T lymphocytes with allogeneic AML blasts or RCC cells. After the third and fourth round of stimulation, responder lymphocytes were monitored daily for expression of the activation-induced antigens CD25, CD69, CD71, CD95, CD134, and CD137 by flow cytometry. With exception of CD137, these markers showed elevated expression levels before rechallenge with leukemia or tumor cells in virtually all cultures (Figure 1) Following restimulation, the expression remained at comparably high levels (CD25, CD69, CD95, CD71) or increased transiently (CD134, CD137) until the next feed with AML/RCC cells. Concerning CD137, maximum expression was consistently observed on day 1 after restimulation. These results were very similar, regardless whether MLLC/MLTC responders were CD4 or CD8 T cells and stimulators were HLA compatible or mismatched, respectively.
Kinetics of activation-induced antigens expressed on allogeneic monoclonal and oligoclonal T-cell cultures. MLLC or MLTC responders were generated by 4 weekly in vitro stimulations of healthy donor CD4 or CD8 T cells with allogeneic AML or RCC cells, respectively. HLA class I and II genotypes of donor and stimulator cells were either fully matched or differed at a single allele or haplotype. CTL clone E77 recognizes mismatch HLA-Cw*0702 on allogeneic RCC cells. Flow cytometry analyses were performed on clonal and oligoclonal T-cell cultures before (day 0) and on days 1 to 6 after rechallenge with AML or RCC cells. Antibodies used were conjugated to FITC (CD4 and CD8) or PE (activation markers). Data represent percentages of T cells with FITC or PE costaining. Similar results were obtained in at least 3 independent experiments per each test condition.
Kinetics of activation-induced antigens expressed on allogeneic monoclonal and oligoclonal T-cell cultures. MLLC or MLTC responders were generated by 4 weekly in vitro stimulations of healthy donor CD4 or CD8 T cells with allogeneic AML or RCC cells, respectively. HLA class I and II genotypes of donor and stimulator cells were either fully matched or differed at a single allele or haplotype. CTL clone E77 recognizes mismatch HLA-Cw*0702 on allogeneic RCC cells. Flow cytometry analyses were performed on clonal and oligoclonal T-cell cultures before (day 0) and on days 1 to 6 after rechallenge with AML or RCC cells. Antibodies used were conjugated to FITC (CD4 and CD8) or PE (activation markers). Data represent percentages of T cells with FITC or PE costaining. Similar results were obtained in at least 3 independent experiments per each test condition.
We also investigated the same panel of activation markers on alloreactive CTL clones recognizing known HLA class I mismatch alleles. Again, a similar expression kinetic was observed after alloantigen challenge (Figure 1). We concluded that CD137 is most suitable for selective allodepletion (SAD) strategies because its expression in MLLC/MLTC (1) shows a consistently low baseline level and (2) increases transiently with a maximum on day 1 after antigen-specific stimulation.
Establishment of the CD137-mediated SAD procedure
We established a 2-step in vitro approach allowing the activation and subsequent CD137-mediated depletion of HLA-mismatch reactive CD8 T cells. First, naive CD8 T lymphocytes were repetitively stimulated in allogeneic MLLC/MLTC against AML/RCC cells mismatched at a single HLA class I allele or haplotype, respectively. The procedure resulted in the in vitro expansion of AML/RCC-reactive CD8 T cells of which a considerable proportion recognized the HLA mismatch allele(s). The antimismatch specificities were activated by stimulating day 21 MLLC/MLTC responders with HLA-negative K562 cells transfected with the disparate HLA class I cDNAs (Figure 2) After 24 hours of incubation, CD137-expressing T cells were depleted using a biotinylated CD137 mAb, anti-Biotin microbeads, and an immunomagnetic depletion column. Previous experiments comparing Biotin- and PE-conjugated reagents showed clearly superior depletion results applying the Biotin strategy (data not shown).
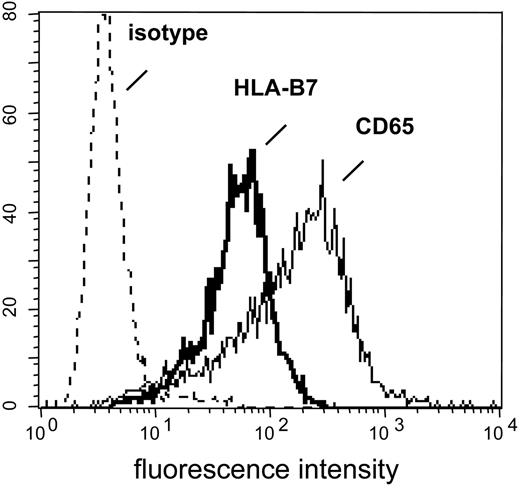
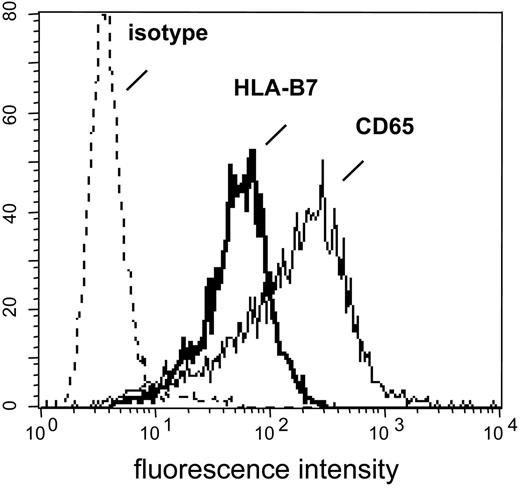
K562-HLA transfectant cells. HLA-negative K562 cells were transfected with cDNAs encoding single HLA class I alleles. After single-cell cloning and expansion in selection medium, K562-HLA cell clones were analyzed by flow cytometry. Monoclonal Abs used recognized either the corresponding HLA allele or myeloid antigen CD65, respectively. Representative data obtained with the K562-HLA-B*0702 cell clone are shown. Comparably strong HLA transgene and CD65 expression levels were found on K562 clones transfected with HLA-A*0201, HLA-A*0301, HLA-B*0801, HLA-B*3501, or HLA-Cw*0702 (data not shown).
K562-HLA transfectant cells. HLA-negative K562 cells were transfected with cDNAs encoding single HLA class I alleles. After single-cell cloning and expansion in selection medium, K562-HLA cell clones were analyzed by flow cytometry. Monoclonal Abs used recognized either the corresponding HLA allele or myeloid antigen CD65, respectively. Representative data obtained with the K562-HLA-B*0702 cell clone are shown. Comparably strong HLA transgene and CD65 expression levels were found on K562 clones transfected with HLA-A*0201, HLA-A*0301, HLA-B*0801, HLA-B*3501, or HLA-Cw*0702 (data not shown).
The CD137-Biotin SAD approach resulted in 2 different fractions: Cells that were retained in the column represented the CD137pos subset. The column effluent contained cells devoid of CD137 expression and was designated the CD137neg subset. However, the latter fraction regularly included K562 transfectant cells potentially capable of stimulating residual antimismatch T cells during the subsequent culture period. This contamination was sufficiently reduced by colabeling day 22 responder lymphocytes with a biotinylated mAb specific for myeloid antigen CD65 that is strongly expressed on K562 cells (Figure 2) but not on T lymphocytes (data not shown).
Anti-CD137 approach efficiently depletes anti–HLA mismatch CD8 T cells
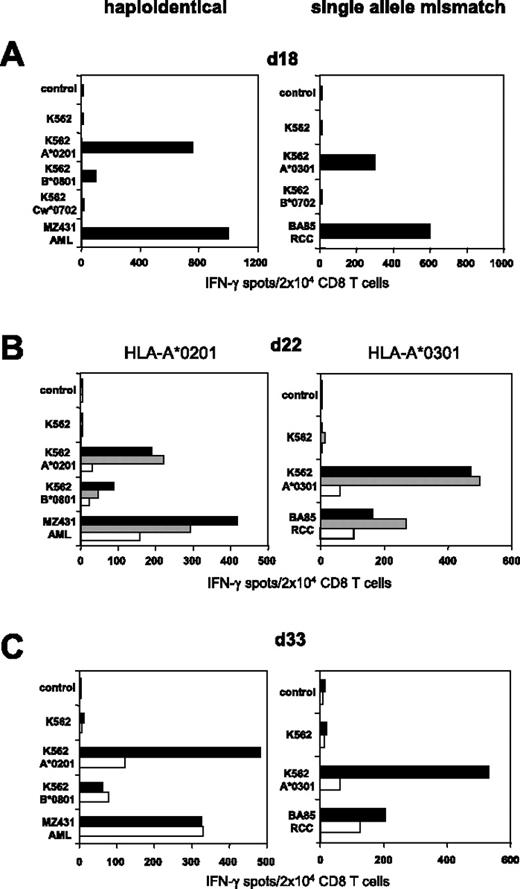
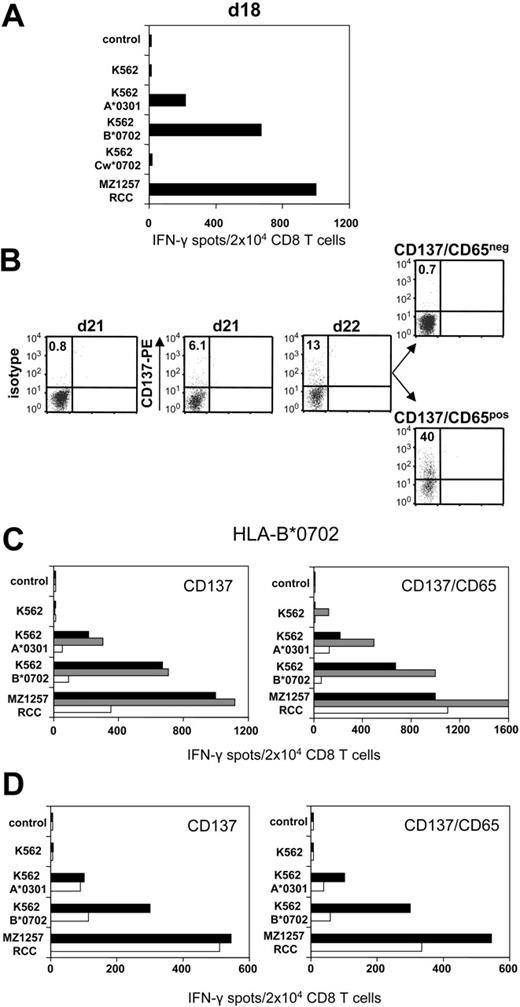
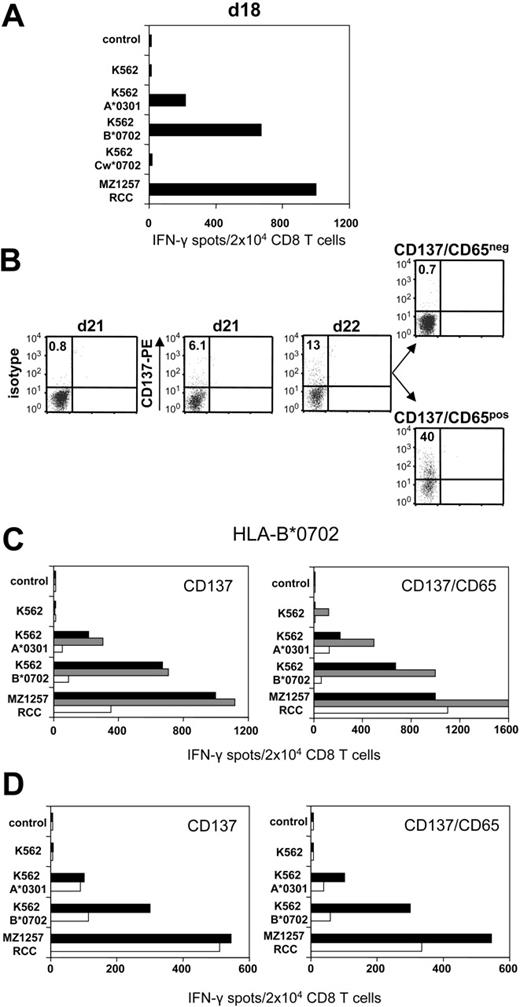
CD8 T cells of healthy donor 203 were stimulated against haploidentical MZ1257-RCC cells in allogeneic MLTC. Day 18 MLTC responder lymphocytes showed strong MZ1257-RCC reactivity which was predominantly directed against the HLA-B*0702 and HLA-A*0301 mismatch alleles (Figure 3A) To activate B*0702-reactive CD8 T cells, day 21 MLTC responders were incubated with K562-HLA-B*0702 transfectant cells. Activated cells were depleted on day 22 using either the CD137 or the CD137/CD65 approach (Figure 3B). The resulting CD137neg fraction showed a strongly reduced frequency of anti-B*0702 mismatch CD8 T cells (ie, 9%) compared with MLTC control cells stimulated exclusively with MZ1257-RCC (Figure 3C). The depletion efficiency was even higher using the CD137/CD65 colabeling strategy. As expected, mismatch-reactive CD8 T cells were enriched in the CD137pos and CD137/CD65pos fractions.
CD137-mediated depletion of HLA-B*0702 mismatch-reactive T cells. CD8 T cells of naive donor 203 were weekly stimulated with haploidentical MZ1257-RCC cells in allo-MLTC (for HLA types see Table 1). (A) IFN-γ ELISPOT results of day 18 MLTC responders tested against MZ1257-RCC and against K562 transfectants expressing the MZ1257-RCC–encoded HLA mismatch alleles A*0301, B*0702, or Cw*0702. (B) After 24 hours of incubation with K562-B*0702 transfectant cells, day 22 MLTC responders were depleted of allo-B*0702–reactive T cells using either the CD137/CD65 or the CD137 approach (latter not shown). Flow cytometry results indicate percentages of CD137pos CD8 T cells. Residual CD137 expression in the CD137/CD65neg subset (ie, 0.7%) was equivalent to day 22 background isotype control (0.7%, not shown). (C) Immediately (day 22) after separation, the CD137neg and CD137/CD65neg fractions (white bars), the CD137pos and CD137/CD65pos fractions (gray bars), and the MLTC control-stimulated exclusively with MZ1257-RCC (black bars) were analyzed for reactivity in IFN-γ ELISPOT assay. (D) The CD137neg, respectively. CD137/CD65neg fractions (white bars) and the MLTC control (black bars) were retested in IFN-γ spot assay on day 33 after simultaneous stimulations with MZ1257-RCC on day 22 and day 29.
CD137-mediated depletion of HLA-B*0702 mismatch-reactive T cells. CD8 T cells of naive donor 203 were weekly stimulated with haploidentical MZ1257-RCC cells in allo-MLTC (for HLA types see Table 1). (A) IFN-γ ELISPOT results of day 18 MLTC responders tested against MZ1257-RCC and against K562 transfectants expressing the MZ1257-RCC–encoded HLA mismatch alleles A*0301, B*0702, or Cw*0702. (B) After 24 hours of incubation with K562-B*0702 transfectant cells, day 22 MLTC responders were depleted of allo-B*0702–reactive T cells using either the CD137/CD65 or the CD137 approach (latter not shown). Flow cytometry results indicate percentages of CD137pos CD8 T cells. Residual CD137 expression in the CD137/CD65neg subset (ie, 0.7%) was equivalent to day 22 background isotype control (0.7%, not shown). (C) Immediately (day 22) after separation, the CD137neg and CD137/CD65neg fractions (white bars), the CD137pos and CD137/CD65pos fractions (gray bars), and the MLTC control-stimulated exclusively with MZ1257-RCC (black bars) were analyzed for reactivity in IFN-γ ELISPOT assay. (D) The CD137neg, respectively. CD137/CD65neg fractions (white bars) and the MLTC control (black bars) were retested in IFN-γ spot assay on day 33 after simultaneous stimulations with MZ1257-RCC on day 22 and day 29.
Repetitive stimulations of the CD137neg and CD137/CD65neg subsets with MZ1257-RCC cells did not restore anti-B*0702 alloreactivity to the original magnitude (Figure 3D). The allo-B*0702–depleted fractions showed a concomitant reduction of anti–HLA-A*0301 mismatch T cells, probably reflecting the sustained activation status of alloreactive T cells within MLTCs (Figure 3C-D). Considering this comprehensive allodepletion, we observed substantial remaining RCC reactivity in the CD137neg and CD137/CD65neg subsets.
Consistent with the IFN-γ ELISPOT results, 51Cr release cytotoxicity testing confirmed that the anti-CD137/CD65 strategy selectively depletes alloreactivity against individual HLA class I mismatch alleles (Figure 4A) To investigate the nature of the residual antitumor response in the allodepleted cell subsets, we performed crossover experiments in which day-21 MLTC responder cells were rechallenged with the original tumor line followed by CD137-mediated depletion. Both the antitumor and the anti–HLA mismatch reactivities were completely removed from the CD137neg cell subset (Figure 4B). This is in contrast to the use of K562-HLA transfectants as allo-APCs where considerable antitumor activity remained after allodepletion (Figure 3C).
Specificity of the anti-CD137 SAD approach. Day 21 MLTC responder cells were obtained by stimulating CD8 T cells of donor 203 against haploidentical MZ1257-RCC cells (for HLA types see Table 1). (A) After 24 hours of incubation with K562-B*0702 transfectant cells followed by anti-CD137/CD65 SAD, the CD137/CD65neg subset, the CD137/CD65pos subset, and undepleted MLTC control cells stimulated exclusively with MZ1257-RCC were analyzed for cytolytic activity against MZ1257-RCC (▪), K562-B*0702 (○), K562-A*0301(▴), and K562 parental cells ([×]) in 51chromium-release assay. (B) After 24 hours of incubation with MZ1257-RCC cells followed by anti-CD137 SAD, the CD137neg fraction (white bars), the CD137pos fraction (gray bars), and the MLTC control stimulated exclusively with MZ1257-RCC (black bars) were analyzed for reactivity in IFN-γ ELISPOT assay. (C) After 24 hours of incubation with K562-B*0702 transfectant cells, allodepletion was performed using anti-CD134/CD65 instead of anti-CD137/CD65 reagents. IFN-γ ELISPOT results are illustrated as described in panel B.
Specificity of the anti-CD137 SAD approach. Day 21 MLTC responder cells were obtained by stimulating CD8 T cells of donor 203 against haploidentical MZ1257-RCC cells (for HLA types see Table 1). (A) After 24 hours of incubation with K562-B*0702 transfectant cells followed by anti-CD137/CD65 SAD, the CD137/CD65neg subset, the CD137/CD65pos subset, and undepleted MLTC control cells stimulated exclusively with MZ1257-RCC were analyzed for cytolytic activity against MZ1257-RCC (▪), K562-B*0702 (○), K562-A*0301(▴), and K562 parental cells ([×]) in 51chromium-release assay. (B) After 24 hours of incubation with MZ1257-RCC cells followed by anti-CD137 SAD, the CD137neg fraction (white bars), the CD137pos fraction (gray bars), and the MLTC control stimulated exclusively with MZ1257-RCC (black bars) were analyzed for reactivity in IFN-γ ELISPOT assay. (C) After 24 hours of incubation with K562-B*0702 transfectant cells, allodepletion was performed using anti-CD134/CD65 instead of anti-CD137/CD65 reagents. IFN-γ ELISPOT results are illustrated as described in panel B.
To obtain additional specificity information on the anti-CD137/CD65 strategy, we replaced the anti-CD137 reagent with a monoclonal antibody recognizing the CD134 antigen that showed a similar expression kinetic on MLLC/MLTC responder cells (Figure 1). Although both procedures were sufficient to deplete anti–HLA mismatch reactive CD8 T cells, enhanced depletion efficiency was obtained with the anti-CD137/CD65 approach (Figures 3C and 4C). A clear disadvantage of the anti-CD134 compared with the anti-CD137 strategy was the higher background expression level of CD134 on MLLC/MLTC responder lymphocytes before depletion (data not shown).
Evaluation in haploidentical and single HLA allele mismatch MLLC/MLTC
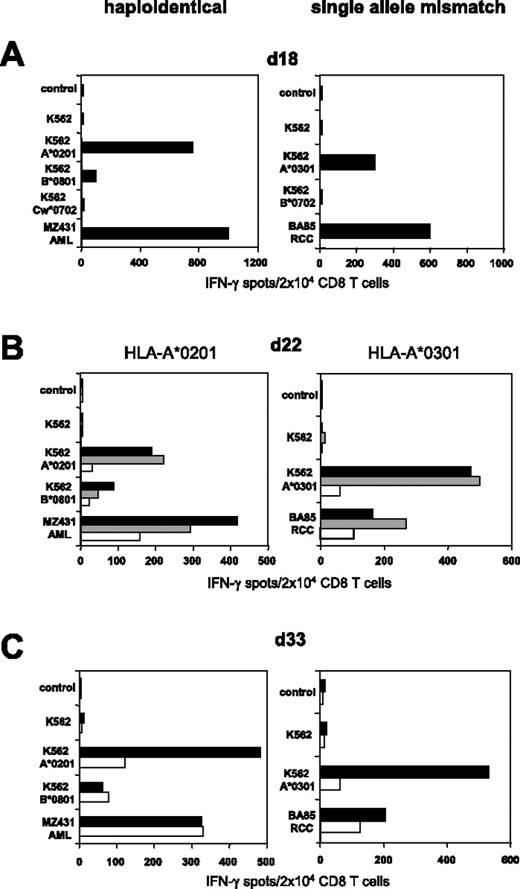
As shown in Figure 3, colabeling with CD65 and CD137 mAbs resulted in superior depletion efficiency if K562-HLA transfectants were used as allogeneic (allo) APCs. We therefore used the CD137/CD65 strategy to deplete alloreactive T cells from further haploidentical MLLCs/MLTCs. Substantial elimination of HLA-A*0201 mismatch reactive T cells was observed in MLLC-stimulating CD8 T cells of healthy donor 308 against haploidentical MZ431-AML blasts (Figure 5A-C). We subsequently explored whether the approach would allow the depletion of alloreactive CD8 T cells arising in single HLA mismatch settings. CD8 T cells of the HLA-A*0302pos donor 410 were stimulated against BA85-RCC cells expressing HLA-A*0301 as the only HLA class I mismatch allele. Functional testing on day 18 demonstrated that a considerable proportion of RCC-reactive MLTC lymphocytes recognized allo-A*0301 (Figure 5A). After a single round of CD137/CD65-mediated depletion, HLA-A*0301 mismatch reactivity was substantially reduced in the CD137/CD65neg subset (ie, 11%; Figure 5B) and did not reach the undepleted control level after repetitive tumor stimulation (Figure 5C).
Efficient SAD in haploidentical and single HLA allele mismatch settings. (Left) CD8 T cells of healthy donor 308 were stimulated with haploidentical MZ431-AML blasts. (Right) CD8 T cells of HLA-A*0302pos healthy donor 410 were stimulated with BA85-RCC cells expressing A*0301 as the only HLA class I mismatch allele (for complete HLA types see Table 1). (A) IFN-γ ELISPOT results of day 18 MLLC/MLTC responders tested against AML/RCC cells and against K562 transfectants expressing the recipients HLA mismatch alleles. (B) Following 24 hours of incubation with K562-A*0201 or K562-A*0301, day 22 MLLC/MLTC responders were depleted of mismatch-reactive CD8 T cells using the CD137/CD65 approach. The resulting CD137/CD65neg fractions (white bars), the CD137/CD65pos fractions (gray bars), and the MLLC/MLTC controls stimulated exclusively with AML/RCC cells (black bars) were tested in IFN-γ spot assay. (C) The CD137/CD65neg fractions (white bars) and the MLLC/MLTC controls (black bars) were reevaluated on day 33 after stimulations with leukemia or tumor cells on day 22 and day 29.
Efficient SAD in haploidentical and single HLA allele mismatch settings. (Left) CD8 T cells of healthy donor 308 were stimulated with haploidentical MZ431-AML blasts. (Right) CD8 T cells of HLA-A*0302pos healthy donor 410 were stimulated with BA85-RCC cells expressing A*0301 as the only HLA class I mismatch allele (for complete HLA types see Table 1). (A) IFN-γ ELISPOT results of day 18 MLLC/MLTC responders tested against AML/RCC cells and against K562 transfectants expressing the recipients HLA mismatch alleles. (B) Following 24 hours of incubation with K562-A*0201 or K562-A*0301, day 22 MLLC/MLTC responders were depleted of mismatch-reactive CD8 T cells using the CD137/CD65 approach. The resulting CD137/CD65neg fractions (white bars), the CD137/CD65pos fractions (gray bars), and the MLLC/MLTC controls stimulated exclusively with AML/RCC cells (black bars) were tested in IFN-γ spot assay. (C) The CD137/CD65neg fractions (white bars) and the MLLC/MLTC controls (black bars) were reevaluated on day 33 after stimulations with leukemia or tumor cells on day 22 and day 29.
Overall, in vitro expansion of AML/RCC-reactive MLLC/MLTC responder cells followed by CD137/CD65-mediated SAD on day 22 was performed in 15 different haploidentical and 1 HLA allele mismatch donor-recipient pairs. The median amplification of tumor-reactive CD8 T-cell responders was 15-fold (range, 5- to 20-fold) between day 0 and day 22. The median cell yield of the allodepleted CD137/CD65neg subset was 33% (range, 28%-44%) of input day-22 MLLC/MLTC cells. The SAD experiments showed that a single round of depletion was sufficient to remove virtually all CD137pos CD8 T cells from the allodepleted cell subsets according to flow cytometry. The frequency of residual CD137pos T cells was generally in the range of background cell numbers stained with the IgG isotype antibody (ie, < 1%). Functionally, the frequency of IFN-γ–secreting anti–HLA mismatch CD8 T cells decreased to a median of 9.5% (range, 1%-16%) compared with undepleted control-cell populations (Table 2)AML/RCC-reactive CD8 T cells persisted in the CD137/CD65neg fractions with a median frequency of 58% (range, 36%-92%) compared with undepleted control cells (Table 2).
K562-HLA transfectants can be replaced by fibroblasts of recipient origin
The integration of K562-HLA transfectants as allo-APCs within the described depletion strategy certainly allows the elimination of T cells recognizing a broad spectrum of allo-HLA binding peptides. However, it is insufficient to deplete T cells that respond to allo-HLA–associated recipient-specific peptides. We therefore explored whether primary fibroblasts presenting the entire recipient HLA mismatch alleles can be implemented as allo-APCs within the CD137 approach. As hematopoietic stimulator cells, we used B lymphoblasts amplified from PBMCs of fibroblast donors by CD40 ligation.
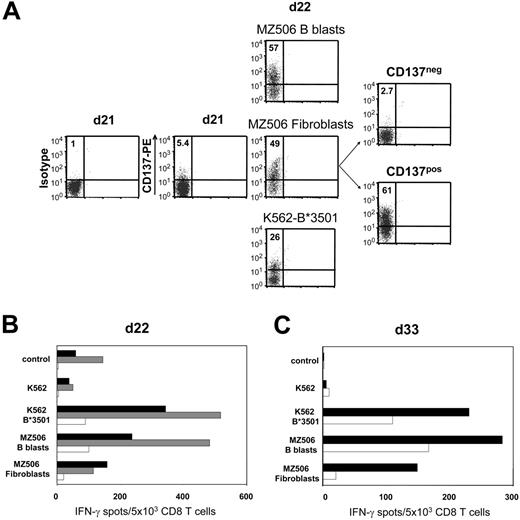
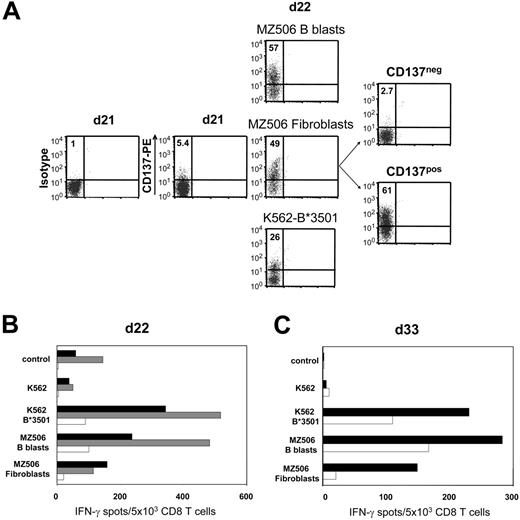
In 1 representative of 3 different experiments, naive CD8 T cells isolated from healthy donor 613 were weekly stimulated against B lymphoblasts of the haploidentical patient MZ506 in allogeneic mixed lymphocyte culture (MLC). Day-18 MLC responders demonstrated stronger reactivity toward patient MZ506-derived B blasts compared with corresponding fibroblasts (data not shown). This reactivity was predominantly directed against the recipient HLA-B*3501 mismatch allele. After a single stimulation with MZ506 fibroblasts on day 21 and subsequent CD137 depletion on day 22, IFN-γ ELISPOT reactivity against fibroblasts was substantially reduced to 13% compared with the undepleted control population (Figure 6A-B) Concomitantly, the B*3501 mismatch reactivity was strongly diminished, confirming the specificity of the approach. The allodepleted CD137neg subset showed a decreased but still significant recognition of MZ506 B blasts. Following repetitive stimulations of the CD137neg subset with MZ506 B blasts, alloreactivity against MZ506 fibroblasts did not return to the level observed in the undepleted control arm (Figure 6C).
Recipient primary fibroblasts can serve as allo-APCs for CD137-mediated SAD. Purified CD8 T cells of healthy donor 613 were stimulated against CD40L-induced B lymphoblasts of patient MZ506 in haploidentical MLC (Table 1). (A) Day 21 MLC responders were incubated with either MZ506-derived B blasts or fibroblasts, or with K562 cells expressing the patient HLA-B*3501 mismatch allele. After 24 hours, the different cell populations were analyzed for CD137 expression by flow cytometry. Only the fibroblast-stimulated day 22 T-cell culture was depleted using the CD137 approach. Numbers indicate percentages of CD137pos CD8 T cells. (B) Immediately (day 22) after separation, the CD137neg fraction (white bars), the CD137pos fraction (gray bars), and the MLC control population stimulated exclusively with MZ506 B blasts (black bars) were tested in IFN-γ ELISPOT assay. (C) The CD137neg fraction (white bars) and the MLC control (black bars) were reevaluated on day 33 after stimulation with MZ506 B blasts on day 22 and day 29.
Recipient primary fibroblasts can serve as allo-APCs for CD137-mediated SAD. Purified CD8 T cells of healthy donor 613 were stimulated against CD40L-induced B lymphoblasts of patient MZ506 in haploidentical MLC (Table 1). (A) Day 21 MLC responders were incubated with either MZ506-derived B blasts or fibroblasts, or with K562 cells expressing the patient HLA-B*3501 mismatch allele. After 24 hours, the different cell populations were analyzed for CD137 expression by flow cytometry. Only the fibroblast-stimulated day 22 T-cell culture was depleted using the CD137 approach. Numbers indicate percentages of CD137pos CD8 T cells. (B) Immediately (day 22) after separation, the CD137neg fraction (white bars), the CD137pos fraction (gray bars), and the MLC control population stimulated exclusively with MZ506 B blasts (black bars) were tested in IFN-γ ELISPOT assay. (C) The CD137neg fraction (white bars) and the MLC control (black bars) were reevaluated on day 33 after stimulation with MZ506 B blasts on day 22 and day 29.
Antiviral CD8 T cells are retained following CD137-mediated allodepletion
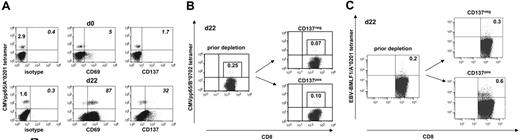
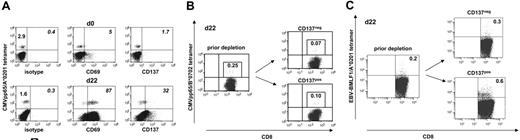
Short-term T-cell cultures contain residual virus-specific memory T cells that might promote antiviral immunity in allograft recipients. These T-cell contaminants can experience nonspecific activation through bystander or cytokine effects. This could lead to their unintended loss during the SAD approach. We investigated the expression of CD137 on CMV-specific T cells isolated from fresh PBMCs and from allogeneic day-22 MLC/MLTC responder populations. Results for CD137 were compared with CD69, which is frequently used in allodepletion approaches. As demonstrated in Figure 7A, less than 10% of CMVpp65 tetramerpos CD8 T cells expressed CD137 or CD69 before in vitro culture. However, the proportion of CD69 or CD137 expressors within CMV tetramerpos T cells increased dramatically in MLC/MLTC responder lymphocytes. CMV-specific CD137pos T cells were always less frequent than their CD69pos counterparts. After a single round of CD137-mediated allodepletion, CMVpp65 tetramerpos CD8 T cells were detected in both the CD137neg and CD137pos cell subsets (Figure 7B). Similar results were obtained for EBV-BMLF1/A*0201-specific CD8 T cells within responder lymphocytes from EBV-seropositive donors (Figure 7C).
CMV and EBV-specific CD8 T cells persist within the allodepleted CD137neg cell subset. (A) Of CMV-seropositive healthy donor 724, fresh PBMCs (day 0) and allo-MLC CD8 responder cells (day 22) were stained with anti-CD8 and with anti-CD69, anti-CD137, or IgG isotype control, respectively, followed by incubation with the CMVpp65/A*0201 tetramer. Donor and MLC stimulator cells were matched for HLA-A*0201. Numbers refer to the percentage of positive cells per total CD8 T cells (left) or per gated tetramerpos CD8 T cells (italics). (B) Of CMV-seropositive healthy donor 837, haploidentical MLTC CD8 responders (day 22) obtained before and after CD137-mediated allodepletion were stained with anti-CD8 and the CMVpp65/B*0702 tetramer. Donor and MLTC stimulator cells were matched for HLA-B*0702. (C) EBV-BMLF1/A*0201 tetramer analysis on day 22 MLTC responders before and after anti-CD137 SAD. The EBV-seropositive healthy donor 203 was matched with haploidentical MLTC stimulator cells for HLA-A*0201. Numbers in panel B and C refer to the percentage of tetramerpos cells per total CD8 T cells with the isotype control subtracted.
CMV and EBV-specific CD8 T cells persist within the allodepleted CD137neg cell subset. (A) Of CMV-seropositive healthy donor 724, fresh PBMCs (day 0) and allo-MLC CD8 responder cells (day 22) were stained with anti-CD8 and with anti-CD69, anti-CD137, or IgG isotype control, respectively, followed by incubation with the CMVpp65/A*0201 tetramer. Donor and MLC stimulator cells were matched for HLA-A*0201. Numbers refer to the percentage of positive cells per total CD8 T cells (left) or per gated tetramerpos CD8 T cells (italics). (B) Of CMV-seropositive healthy donor 837, haploidentical MLTC CD8 responders (day 22) obtained before and after CD137-mediated allodepletion were stained with anti-CD8 and the CMVpp65/B*0702 tetramer. Donor and MLTC stimulator cells were matched for HLA-B*0702. (C) EBV-BMLF1/A*0201 tetramer analysis on day 22 MLTC responders before and after anti-CD137 SAD. The EBV-seropositive healthy donor 203 was matched with haploidentical MLTC stimulator cells for HLA-A*0201. Numbers in panel B and C refer to the percentage of tetramerpos cells per total CD8 T cells with the isotype control subtracted.
HLA tetramer results were confirmed by IFN-γ ELISPOT assays analyzing allodepleted and undepleted T-cell populations for reactivity against HLA class I binding CMVpp65 and EBV-BMFL1 peptide epitopes (data not shown). Overall, the frequency of virus-specific CD8 T cells persisted in the CD137neg subsets with a median percentage of 78% (range, 24%-150%) compared with the level observed in undepleted controls (Table 2).
Discussion
Two SAD approaches that are based on the induction of anergy by B7-CD28 costimulatory blockade17 and the removal of alloreactive T cells by anti-CD25 immunotoxin32,33 have been clinically validated in haploidentical transplantation. In these trials transfusion of allodepleted donor peripheral blood or bone marrow cells resulted in improved T-cell reconstitution32,33 and low incidence of GVHD but was unable to prevent leukemia relapse in several patients. To address this problem we designed an alternative approach in which graft-versus-malignancy (GVM) reactivity is enriched by repetitive leukemia/tumor in vitro stimulations prior to CD137-mediated SAD. The rationale for this procedure was derived from observations in viral disease models in which the adoptive transfer of in vitro–generated CMV and EBV reactive donor T-cell lines was followed by significant antiviral activity and a remarkably low rate of GVHD, even in unrelated and HLA mismatch donor-recipient pairs.45-47 The reduced GVHD incidence might be explained by a spontaneous depletion of antirecipient reactive T cells through “death-by-neglect” during prolonged culture periods.48 We assume that a considerable part of these allospecificities (eg, against liver or gut-specific recipient antigens) are neither detected nor depleted by current readout and SAD instruments.
According to flow cytometry analysis the anti-CD137 approach effectively depleted CD137-expressing cells below background expression levels. This result corresponds favorably to flow cytometry data obtained with the anti-CD25 immunotoxin technology.26 Functional assays demonstrated that the anti-CD137 method reduced the frequency of alloreactive T cells by 1 log, which seems less potent compared with the B7-CD28 costimulatory blockade (ie, 1-4 log) and the anti-CD25 (ie, 0% to 5% of predepletion level) strategies.17,32,33 However, comparing these allodepletion levels is extremely difficult because several methodologic differences have to be considered. First, previous studies have measured the antihost proliferative response following a culture period of 3 to 6 days after depletion, whereas we determined alloantigen-induced IFN-γ secretion at the single-cell level immediately after depletion without any preculture period. Further differences included the origin of allo-APCs (ie, hematopoietic versus nonhematopoietic), the rechallenge with allo-APCs during the ELISPOT assay period, and the quality of responder cells (ie, 1-2 rounds of stimulation versus at least 4 rounds of stimulation). Particularly, repetitive stimulations of responder cells with allogeneic targets could lead to an increased visualization of low-affinity alloreactive T cells that are less prone to elimination due to a low CD137 expression level. Such T-cell populations may stay undetected using only 1 to 2 allo-APC stimulations in previous SAD approaches. With all these differences it remains unclear whether the anti-CD137 method can translate into an equivalent GVHD reduction compared with already established SAD technologies after transfusion of allodepleted cells in vivo. To address this question we are currently investigating the CD137-mediated SAD strategy in a murine P (H-2b) → F1 (H-2bxd) adoptive transfer model in vivo. Preliminary data demonstrate a reduced GVHD incidence following infusion of allodepleted T-cell lines compared with undepleted controls (U.F.H., unpublished results, July 24, 2006).
Repeated in vitro stimulations with leukemia or tumor cells have the advantage of expanding tumor-reactive T-cell precursors that are present at very low frequency in naive PBMCs.49,50 However, extending the culture period over several weeks carries the risk of generating late-stage effector T cells that lose antitumor efficacy in vivo.51 Encouraging clinical results have been observed in adoptive immunotherapy trials using short-term cultured T-cell lines, either generated from stem-cell donors against CMV and EBV45-47 or isolated autologously from patients with melanoma.52 Altogether, these studies favor the use of oligoclonal T-cell populations that are enriched for GVM reactivity during a preferably short culture period.
Most SAD approaches have been performed after primary MLC in which donor PBMCs were stimulated against recipient APCs over 1 to 7 days. The allo-APCs were mainly derived from hematopoietic cells.23,26,53,54 Although such a strategy eliminates T cells directed against major and minor histocompatibility antigen disparities ubiquitously expressed by the recipient, T cells recognizing nonhematopoietic tissue-specific antigens are largely spared. The latter might destroy normal epithelial tissues, predominantly of the skin, gut, and liver. A further caveat to the application of hematopoietic allo-APCs is that it can reduce beneficial donor antihost immune reactivity, including GVM and graft-versus-recipient hematopoiesis effects.55 In a different SAD procedure, van Dijk et al24 successfully used recipient keratinocytes as epithelial allo-APCs. However, isolating primary epithelial cells from all potential GVHD target organs of the recipient seems infeasible.
Stimulating donor T cells against HLA disparate tumor cells triggers alloreactivity against foreign major histocompatibility antigens expressed by the recipient malignancy. Applied in vivo, such alloreactive T cells might cause serious inflammation and destruction of HLA-bearing normal host tissues. We demonstrate herein that HLA-negative K562 cells transfected with single recipient HLA class I alleles can be applied for depleting anti–class I CD8 T cells. Most notably, K562-HLA transfectants were an excellent tool to visualize alloreactive T cells specifically for individual HLA alleles. In several experiments, HLA-mismatch reactive T cells demonstrated increased IFN-γ spot production when K562-HLA transfectants rather than the original stimulator cells were used as APCs. This might be explained by the superior APC function of K562-HLA cells, which express high levels of HLA class I molecules (Figure 2) usually not found on tumor cells. In HLA-incompatible stem cell transplantation (SCT), K562-HLA reagents might help to screen donor PBMCs or T-cell products regarding potential alloreactivity. For an “off-the-shelf” use, we have already generated more than 20 different K562 cell clones each expressing a single HLA class I allele.
In addition to major histocompatibility antigen disparities, alloreactive T cells recognize polymorphic minor histocompatibility (minor H) antigens that are presented by HLA molecules shared between the donor and recipient.56 Minor H antigens are either expressed specifically in individual tissues or are broadly expressed in various tissue lineages. Particularly, the latter are regarded as important target structures of GVHD-inducing T cells.57 Activation and subsequent depletion of these specificities require recipient APCs that carry a preferably comprehensive set of ubiquitous minor H antigens. Our results suggest the use of primary fibroblasts for this purpose. As a clear advantage over other nonhematopoietic recipient cells, they can be reliably expanded from skin biopsies in vitro. Fibroblasts express HLA class I as well as ubiquitous minor H antigens.58,59 After IFN-γ treatment, fibroblasts up-regulate expression of CD40, CD54, CD58, HLA class I and II, allowing concomitant activation of alloreactive CD4 T cells (M.N., unpublished observations, July 20, 2006). Unlike leukemia models, stimulating donor T cells against RCC cells might also result in effector cells crossreacting against antigens shared between different host epithelial tissues. To deplete antiepithelium T-cell specificities, recipient-derived proximal tubular epithelial cells rather than fibroblasts could serve as allo-APCs. The former can be readily isolated from adjacent normal kidney tissue of tumor nephrectomy samples by short-term in vitro culture.60
Our results show the persistence of CMV and EBV-reactive CD8 T cells in the allodepleted CD137neg cell subset. In single experiments virus-reactive CD8 T cells were even enriched in the allodepleted fractions compared with undepleted control populations. Similar findings have been reported by investigators using the anti-CD25 SAD approach.25,26 This observation might be explained by a more robust nature of virus-specific memory CD8 T cells that can up-regulate antiapoptotic genes such as Bcl-2.61,62 Increased Bcl-2 expression may result in a survival advantage over other MLLC/MLTC cells during the stressful passage through the allodepletion columns.
We further show that the activation-induced antigen CD137 is most suitable for SAD of established T-cell lines due to low interference with nonspecific cytokine effects and bystander activation (Figure 1). Interestingly, the frequency of CD137pos T cells assessed by flow cytometry at 24 hours after alloantigen stimulation corresponded favorably with the number of IFN-γ spot-forming T cells determined after a 20-hour stimulation period in ELISPOT plates. Because of the uniform expression kinetic following T-cell activation, the CD137 marker might be also suitable to enrich antigen-specific T cells within lymphocyte bulk cultures. Such an approach would be a less laborious alternative to cytokine secretion assays that select specific T cells in a complex multistep procedure.63
Our study demonstrates the feasibility of generating leukemia/tumor-reactive CD8 T-cell lines followed by CD137-mediated SAD in HLA haploidentical and single allele mismatch settings. The similar CD137 expression kinetic observed in HLA-matched MLLC/MLTC CD8 responders (Figure 1) suggests that this strategy might be also suitable for depleting alloreactivity in HLA-compatible minor H antigen disparate donor-recipient pairs. We further introduce HLA-transfected K562 cells as well as primary fibroblasts as allo-APCs within the SAD procedure. Ongoing studies in mice investigate GVHD and GVM responses of allodepleted CD137neg T-cell populations following adoptive transfer in vivo. We also initiated a project aiming toward the large-scale production of AML-reactive allodepleted T-cell lines in the HLA haploidentical setting under good manufacturing practice conditions. The protocol includes the development of all cell cultures in serum-free media and the use of fresh AML blasts as stimulator cells and primary patient fibroblasts as allo-APCs. If successfully completed, we are planning a pilot trial in haploidentical transplantation to investigate whether the infusion of AML-driven anti-CD137 allodepleted T-cell lines confers strengthened GVL reactivity without causing GVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: W.H. and U.F.H. designed the research; M.N., T.C.W., B.B., M.T., I.L., and S.A.K. performed the research; T.C.W., C.M.B., M.G., and R.G.M. provided vital reagents; M.N. and T.C.W. analyzed the data; W.H., T.C.W., M.N., U.F.H., and C.H. wrote paper.
T.C.W. and M.N. contributed equally to this work. U.F.H. and W.H. are joint senior authors.
Acknowledgments
We thank Cornelia Metz and Elke Schnürer for their technical assistance.
This work was supported by the “Deutsche Krebshilfe” (grant no. 70-2428-IIA and IVB) (U.F.H. and W.H.), and the “Stiftung Rheinland-Pfalz für Innovation” (grant no. 15202-386261/684) to (U.F.H. and W.H.).

![Figure 4. Specificity of the anti-CD137 SAD approach. Day 21 MLTC responder cells were obtained by stimulating CD8 T cells of donor 203 against haploidentical MZ1257-RCC cells (for HLA types see Table 1). (A) After 24 hours of incubation with K562-B*0702 transfectant cells followed by anti-CD137/CD65 SAD, the CD137/CD65neg subset, the CD137/CD65pos subset, and undepleted MLTC control cells stimulated exclusively with MZ1257-RCC were analyzed for cytolytic activity against MZ1257-RCC (▪), K562-B*0702 (○), K562-A*0301(▴), and K562 parental cells ([×]) in 51chromium-release assay. (B) After 24 hours of incubation with MZ1257-RCC cells followed by anti-CD137 SAD, the CD137neg fraction (white bars), the CD137pos fraction (gray bars), and the MLTC control stimulated exclusively with MZ1257-RCC (black bars) were analyzed for reactivity in IFN-γ ELISPOT assay. (C) After 24 hours of incubation with K562-B*0702 transfectant cells, allodepletion was performed using anti-CD134/CD65 instead of anti-CD137/CD65 reagents. IFN-γ ELISPOT results are illustrated as described in panel B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-04-014100/4/m_zh80010705620004.jpeg?Expires=1768058846&Signature=5IXViApEZRXl9NtzvpNfb4DYbflbX3Es6agoO4XuuNIf3wbbqEV-7l0wLNirkbOiq5svWwGfD0CmmZJFR4iell8rX2i7Qlh8jnAPY0PAGOSOJjmcwhBcDrz6UdSuFrILgCohG9KsJAZBNWKqrYWlnAqNbRohnkdL4jbRdbEvuSyc75xU8RvQe9DFgUz~Hfg9eruKG0zDXLAe-6vkVYDUMcpQBfL9ijYss84Qdb35-yysa8rKmUYRcUY1ULjpUrYMiFVZzt4jP9eELdBnOgG6hYoodwEEMeuTes7Lebn5J6UkdLH-Sf1TCpZj8DptMX0~tIES10VFucaAXPmstM42Iw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. Specificity of the anti-CD137 SAD approach. Day 21 MLTC responder cells were obtained by stimulating CD8 T cells of donor 203 against haploidentical MZ1257-RCC cells (for HLA types see Table 1). (A) After 24 hours of incubation with K562-B*0702 transfectant cells followed by anti-CD137/CD65 SAD, the CD137/CD65neg subset, the CD137/CD65pos subset, and undepleted MLTC control cells stimulated exclusively with MZ1257-RCC were analyzed for cytolytic activity against MZ1257-RCC (▪), K562-B*0702 (○), K562-A*0301(▴), and K562 parental cells ([×]) in 51chromium-release assay. (B) After 24 hours of incubation with MZ1257-RCC cells followed by anti-CD137 SAD, the CD137neg fraction (white bars), the CD137pos fraction (gray bars), and the MLTC control stimulated exclusively with MZ1257-RCC (black bars) were analyzed for reactivity in IFN-γ ELISPOT assay. (C) After 24 hours of incubation with K562-B*0702 transfectant cells, allodepletion was performed using anti-CD134/CD65 instead of anti-CD137/CD65 reagents. IFN-γ ELISPOT results are illustrated as described in panel B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-04-014100/4/m_zh80010705620004.jpeg?Expires=1768088016&Signature=aLknXwT4y~XWHH47DeP2vdva~DBFJVR1p0SAmPn7D7ClXmv6nd2YqVPaVQcaSjx8e6PEevilg6OcQnF1M9sQv1FSs9pnZj0030WZVgNHx~~sxY5tSoCOGQoc6D6W-r79FFXHX3h2RkBdW3BG0Sw~iAwEFqk5MCixq4UiL2COdpNJyYHSF3-BRjlTYOzVcfH4HpESDrCuiUHJubqiHLrLRfkxxoLsyavmTOMGXZfaRDag7xc0Pmfp8r9VILkMwMhvxGfKEmwQBUgVcNh-7I6iT-XEuFc9Acz147csdDzkZIgCih6uDg6xQR~M2LipiJ7W-u8RU6xBupWj7HZVu3GjZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)