Abstract
CD33-related Siglecs (CD33rSiglecs) are a family of sialic acid–recognizing lectins on immune cells whose biologic functions are unknown. We studied in vivo functions of Siglec-F, the CD33rSiglec expressed on mouse eosinophils, which are prominent in allergic processes. Induction of allergic lung inflammation in mice caused up-regulation of Siglec-F on blood and bone marrow eosinophils, accompanied by newly induced expression on some CD4+ cells, as well as quantitative up-regulation of endogenous Siglec-F ligands in the lung tissue and airways. Taken together with the tyrosine-based inhibitory motif in the cytosolic tail of Siglec-F, the data suggested a negative feedback loop, controlling allergic responses of eosinophils and helper T cells, via Siglec-F and Siglec-F ligands. To pursue this hypothesis, we created Siglec-F–null mice. Allergen-challenged null mice showed increased lung eosinophil infiltration, enhanced bone marrow and blood eosinophilia, delayed resolution of lung eosinophilia, and reduced peribronchial-cell apoptosis. Anti–Siglec-F antibody cross-linking also enhanced eosinophil apoptosis in vitro. These data support the proposed negative feedback role for Siglec-F, represent the first in vivo demonstration of biologic functions for any CD33rSiglec, and predict a role for human Siglec-8 (the isofunctional paralog of mouse Siglec-F) in regulating the pathogenesis of human eosinophil-mediated disorders.
Introduction
Siglecs are vertebrate lectins recognizing sialic acid (Sia)–containing glycans.1,2 More than a dozen human Siglecs are reported, of which Siglec-3 and Siglecs-5 through -11 are classified into a subgroup named CD33-related Siglecs (CD33rSiglecs), which are rapidly evolving.1–4 Although each CD33rSiglec has unique expression profile, they are predominantly found on leukocytes involved in innate immunity.
Siglecs are single-pass type I transmembrane proteins. A conserved arginine residue in the N-terminal Ig-like V-set domain is required for optimal Sia recognition. Most CD33rSiglecs also have 2 putative tyrosine-based signaling motifs in their cytoplasmic tails, one of which conforms to an immunoreceptor tyrosine-based inhibitory motif (ITIM).5 In vitro experiments showed phosphorylation of these tyrosine residues, with recruitment of tyrosine phosphatases.6–9 Antibody-mediated cross-linking of some CD33rSiglecs results in inhibition of cell proliferation and function, and/or induction of apoptosis.10–13 While these in vitro data suggest that CD33rSiglecs are inhibitory signaling molecules that dampen immune-cell functions, in vivo proof is lacking. Anti-Siglec antibodies also tend to induce rapid endocytic clearing of the cognate Siglec from cell surfaces,14,15 complicating interpretation of the observed effects. We previously reported analysis of mice deficient for CD33, finding minimal phenotypes.16 However, this model was not ideal to study in vivo functions of typical CD33rSiglecs, as mouse CD33 lacks an ITIM in the cytosolic tail.
Siglec-F is a CD33rSiglec prominently expressed on mature circulating mouse eosinophils, and on some myeloid precursors in bone marrow.17,18 It has a binding preference for α2-3–linked Sias,18 with the best-known ligand being 6′sulfo-sialyl-Lewis X.19 Of interest, this structure is also the preferred ligand for human Siglec-8,20 a molecule also specifically expressed on human eosinophils.21,22 Although mouse Siglec-F is not the true ortholog of human Siglec-8,18 their marked similarities in expression patterns and ligand preferences suggest that they play equivalent roles. Studying Siglec-F in a mouse model should therefore provide general insights into the currently unknown biologic roles of typical CD33rSiglecs with ITIMs, as well as about the physiological functions of Siglec-8 in human eosinophils, and in eosinophil-mediated diseases.
The elevated eosinophil count in allergic conditions is well known,23,24 as is a critical role for CD4+ Th2 cells in regulating allergic inflammatory responses involving eosinophils.25–27 We investigated the biologic roles of Siglec-F in vivo, using wild-type (WT) and Siglec-F–null mice in an induced lung allergic response model associated with blood and bone marrow eosinophilia, tissue eosinophil accumulation, and mediator release.28,29 This model also mimics some other features of bronchial asthma in humans, such as IgE-mediated mast-cell activation and degranulation, airway inflammation and hyperreactivity, CD4+ T-cell infiltration and cytokine production, goblet-cell hyperplasia, and mucus overproduction.30 Our data with WT mice using this model suggested a negative feedback loop involving Siglec-F in controlling eosinophilic responses, a hypothesis confirmed by studies of Siglec-F–null mice. These results represent the first demonstration of an in vivo biologic role for a CD33rSiglec, and also reveal an unexpected potential role for CD33rSiglecs in regulating T-cell induction of eosinophilic responses.
Materials and methods
Mice
C57BL/6 mice were kept in a pathogen-free, limited-access barrier facility. Siglec-F–null mice were generated as described in the Supplemental Methods (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Mice that are 8 to 10 weeks old were used in experimental protocols approved by the UCSD Institutional Animal Care and Use Committee.
Reagents
Rabbit antibody against mouse major basic protein (MBP) was a gift from Dr James Lee (Mayo Clinic Arizona, Scottsdale, AZ). R-phycoerythrin (PE)–conjugated rat anti–mouse Siglec-F (clone E50-2440) was derived from a hybridoma clone prepared in collaboration with BD Biosciences Pharmingen (San Diego, CA).18 The following materials were obtained from the sources indicated: hamster anti–mouse CD3 (clone 145-2C11) and hamster anti–mouse CD28 (clone 37.51): BD Biosciences Pharmingen; TriColor-conjugated anti–mouse CD4 (clone CT-CD4) and anti–mouse CD8a (clone 5H10), Innitrogen (Carlsbad, CA); rat anti–mouse CD4 (clone GK 1.5), Chemicon (Temecula, CA); and fluorescein-conjugated rat anti–mouse CCR3 (clone 83101), R&D Systems (Minneapolis, MN). PE- or fluorescein-conjugated rat IgG2a κ isotype (clone R35-95) was from BD Biosciences Pharmingen and served as the isotype-matched control antibodies.
Induction of allergic airway inflammation in mice
Pulmonary eosinophilia in mice was induced as previously described.31 In brief, mice were sensitized by intraperitoneal injections on days 0 and 12 with 50 μg ovalbumin (OVA, grade V) adsorbed to 1 mg alum (both from Sigma-Aldrich, St Louis, MO) in 200 μL phosphate-buffered saline (PBS). Intranasal OVA challenges (20 μg OVA in 50 μL PBS) were administered on days 24, 26, and 28 under isoflurane anesthesia. A control group was sensitized with OVA and then challenged with PBS, in place of OVA. On day 29, 24 hours after the last OVA challenge, mice were examined for airway responsiveness and airway inflammation. Cell counts in blood smears and tissues were done in a blinded fashion.
Airway inflammation resolution assay
Mice were sensitized and intranasally challenged with OVA. One group of mice was examined one day after the last challenge, and another group 7 days after the last challenge, to look for the extent of resolution of inflammation.
Eosinophil counts in peripheral blood, bronchoalveolar lavage, and bone marrow
Peripheral blood was collected from mice by cardiac puncture into EDTA-containing tubes. Erythrocytes were lysed using a 1:10 solution of 100 mM potassium carbonate–1.5 M ammonium chloride. The remaining cells were resuspended in 1 mL PBS. Bronchoalveolar lavage (BAL) was collected by lavaging the lung with 1 mL PBS via a tracheal catheter.31 BAL was centrifuged, supernatant was collected and frozen at −80°C, and cells were resuspended in 1 mL PBS. Bone marrow cells were flushed from femurs with 1 mL PBS, centrifuged, and resuspended in 1 mL PBS. Total leukocytes were counted using a hemocytometer. To perform differential cell counts, 200 μL resuspended BAL cells, peripheral-blood leukocytes, or 20 μL bone marrow cell suspensions was cytospun onto microscope slides and air-dried. Slides were stained with Wright-Giemsa and differential cell counts were performed under a light microscope.
Lung tissue eosinophil counts
Lungs were inflated with an intratracheal injection of 4% paraformaldehyde solution, left overnight at 4°C, and then embedded in paraffin, using standard procedures. They were sectioned at 5 μm onto slides,31 deparaffinized, and hydrated. Endogenous peroxidase activity was quenched in 3% H2O2/methanol for 10 minutes. Sections were digested with pepsin for 10 minutes at 37°C, rinsed, and blocked for 30 minutes in goat serum in PBS. Slides were incubated with rabbit anti–mouse MBP (1:500) overnight in a moist chamber at 4°C. VECTASTAIN Rabbit ABC kit and AEC (3-amino-9-ethyl carbazole) reagent (Vector Laboratories, Burlingame, CA) were used to detect immunoreactivity. Sections were counterstained with hematoxylin and mounted with aqueous mounting media. Peribronchial eosinophil counts were taken under a light microscope and 8 to 10 bronchi/slide were counted.
Cell apoptosis assays
Terminal transferase dUTP nick-end labeling (TUNEL) assays were performed to detect apoptotic cells in lung sections with an ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Intergen, Purchase, NY) following the manufacturer's instruction. After Methyl green (Vector Laboratories) counterstaining, apoptotic cells in the bronchus and peribronchial area were counted using light microscopy at 40 × magnification. At least 10 medium-sized bronchi were examined for each sample.
Effects of cross-linking an anti–Siglec-F antibody on mouse eosinophils
Mouse eosinophils were purified from IL-5 transgenic mice (> 95% purity) and incubated in media alone, or with anti–Siglec-F antibody (2.5 μg/mL), with or without a secondary cross-linking anti–rat IgG1/2a Ab (2.5 μg/mL; BD Biosciences Pharmingen) for 24 hours in a CO2 incubator. The percentage of TUNEL-positive eosinophils was then determined on cytospin slide preparations.
In vitro activation of T cells
Mononuclear cells were isolated from peripheral blood or spleen by Ficoll-Paque centrifugation, washed with PBS, and resuspended in RPMI 1640 media supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin sulfate. The cells (0.5 × 106/well) were transferred to 48-well plates precoated with anti–mouse CD3 (2 μg/well) and anti–mouse CD28 (1 μg/well), and cultured for 3 days.
Flow cytometric analysis of Siglec-F
Leukocytes from blood or spleen or in vitro–activated T cells were incubated with anti–mouse CD16/CD32 to block FcγIII/II receptor. Each sample (1 × 105 cells) was then stained with anti–mouse Siglec-F-PE and anti–mouse CCR3-fluorescein, anti–mouse CD4-TriColor, or anti–mouse CD8-TriColor, and subjected to flow cytometric analysis. FACSCalibur (BD Biosciences Immunocytometry Systems, San Jose, CA) and Flowjo software (Tree Star, Ashland, OR) were used to collect and analyze the data.
Detection of Siglec-F and CD4 in lung sections
Paraffin-embedded lung sections were rehydrated, quenched with 3% H2O2/methanol for 1 hour, and subjected to antigen retrieval (5 minutes × 2 in a microwave). Sections were then blocked and immunostained with rat anti–mouse Siglec-F (1:20) at 4°C overnight, followed by biotinylated goat anti–rat IgG (1:200) for 1 hour, and peroxidase-conjugated streptavidin (1:100) for 1 hour. Using a TSA kit (Molecular Probes, Eugene, OR), slides were incubated with Tyramide-Alexa555 in 0.0015% H2O2 for 10 minutes. Slides were washed and blocked again. CD4 staining was performed with rat anti–mouse CD4 (1:1000) at 4°C overnight, followed by biotinylated goat anti–rat IgG (1:200) for 1 hour, and streptavidin-dichlorotriazinylamino fluorescein (1:300; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour. Sections were scanned using a Nikon Eclipse E800 microscope (Nikon, Tokyo, Japan). Images were captured and analyzed using Microfire (Olympus America, Center Valley, PA). Images were overlaid using Photoshop software (Adobe Systems, San Jose, CA).
Probing of lung sections for Siglec-F ligands
Cryostat sections of lung tissues were air-dried, fixed in acetone for 10 minutes, quenched in 0.03% H2O2/methanol for 30 minutes, and blocked for endogenous biotin. Slides were incubated with recombinant Siglec-F-Fc or R114A Siglec-F-Fc,18 followed by biotin-conjugated goat anti–human IgG (1:750; Vector Laboratories) for 30 minutes, peroxidase-conjugated streptavidin (1:500; Jackson ImmunoResearch Laboratories) for 30 minutes, and Vector NovaRed (Vector Laboratories) for 40 minutes. Slides were counterstained with Mayer hematoxylin.
Quantitative analysis of Siglec-F-Fc–immunostained epithelium and peribronchial cells
Lungs from OVA- and non–OVA-challenged WT mice (n = 2 each) were used for Siglec-F-Fc immunostaining, and subjected to quantitative image analysis. Following lung immunostaining, the area of epithelial Siglec-F-Fc immunostaining was outlined and quantified using a light microscope (Leica DMLS; Leica Microsystems, Depew, NY) attached to an image-analysis system (Image-Pro Plus: MediaCybernetics, Silver Spring, MD). Results are expressed as the area of epithelial immunostaining per micrometer length of epithelial basement membrane of bronchioles with 150 to 250 μm internal diameter. The number of individual nonepithelial cells in the peribronchial space that immunostained positive for Siglec-F-Fc was also counted using a light microscope. Results are expressed as the number of peribronchial cells immunostained per bronchiole. At least 10 bronchioles were counted in each slide.
Evaluation of BAL mucin production by Sia quantification
BAL (20 μL) was mixed well with methanol and chloroform at a 1:10:10 ratio, and centrifuged to extract lipids. The protein-containing pellet was air-dried, and mucin fragments were isolated based on modification from our recent method for isolating carcinoma mucins.32 See Document S1 for details.
Airway responsiveness to methacholine
Airway responsiveness to methacholine was assessed 24 hours after the final OVA challenge in intubated and ventilated mice as described.33 See Document S1 for details.
Statistical analyses
Results from the different groups were compared by 2-tailed Student t test using a statistical software package (In Stat; GraphPad Software, San Diego, CA). All results are given as mean ± standard error of the mean. P values of less than .05 were considered statistically significant.
Results
Eosinophil Siglec-F expression is up-regulated in a lung allergy model
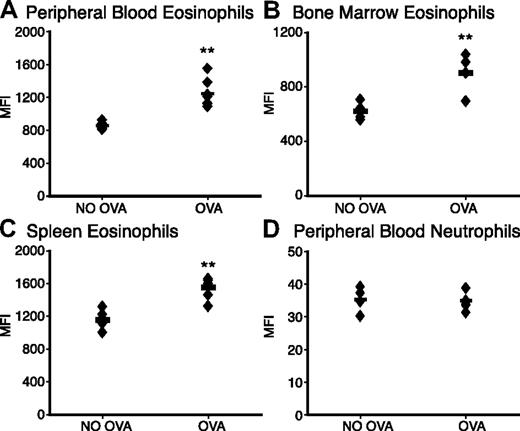
To evaluate the role of Siglec-F on eosinophils and in eosinophil-mediated diseases, we used a murine asthmalike lung allergy model, in which eosinophils are recruited from the bone marrow to the lung. Mice were sensitized by repeated intraperitoneal injection of chicken ovalbumin (OVA), followed by intranasal challenge with the same antigen. Notably, eosinophils from blood, bone marrow, and the spleen showed significantly increased Siglec-F levels after OVA challenge (Figure 1A-C). In contrast, neutrophils did not show any increase in their low levels of Siglec-F (Figure 1D, note that the Y-axis scale is 100-fold lower for neutrophils).
Eosinophil Siglec-F expression level is up-regulated upon OVA challenge. WT mice with OVA sensitization and challenge (OVA) were compared with OVA-sensitized and PBS-challenged control mice (NO OVA) for Siglec-F expression. Leukocytes from (A) peripheral blood, (B) bone marrow, or (C) spleen were stained with anti-CCR3 and anti–Siglec-F (or a control antibody). Cells were analyzed by flow cytometry and data plotted as the median fluorescence intensity (MFI) of anti–Siglec-F staining. (D) Peripheral-blood neutrophils serve as a control to show the specific change in eosinophil Siglec-F expression (note the different Y-axis, indicating that the expression levels on neutrophils are also much lower). Histogram profiles were unimodal, making the MFI a valid means of presenting the comparisons (n = 6; individual mice shown as ♦; averages shown as bars; data shown are representative of 3 experiments). **P < .01.
Eosinophil Siglec-F expression level is up-regulated upon OVA challenge. WT mice with OVA sensitization and challenge (OVA) were compared with OVA-sensitized and PBS-challenged control mice (NO OVA) for Siglec-F expression. Leukocytes from (A) peripheral blood, (B) bone marrow, or (C) spleen were stained with anti-CCR3 and anti–Siglec-F (or a control antibody). Cells were analyzed by flow cytometry and data plotted as the median fluorescence intensity (MFI) of anti–Siglec-F staining. (D) Peripheral-blood neutrophils serve as a control to show the specific change in eosinophil Siglec-F expression (note the different Y-axis, indicating that the expression levels on neutrophils are also much lower). Histogram profiles were unimodal, making the MFI a valid means of presenting the comparisons (n = 6; individual mice shown as ♦; averages shown as bars; data shown are representative of 3 experiments). **P < .01.
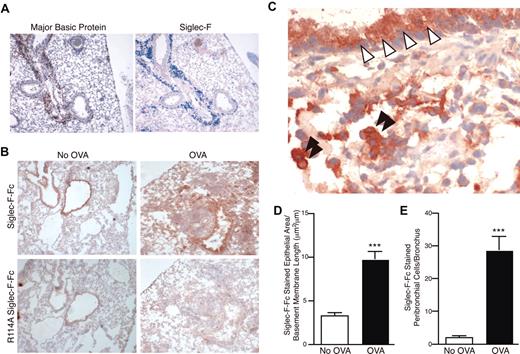
Tissue sections of lungs of unchallenged mice showed very little staining with anti–Siglec-F antibodies (data not shown; some alveolar and tissue macrophages were positive). In contrast, staining of the inflamed lungs from the OVA-challenged mice showed a marked infiltration with Siglec-F–positive cells, especially in the peribronchial areas (Figure 2A). This staining overlapped with that for major basic protein (MBP), a specific eosinophil marker (Figure 2A).
Siglec-F and sialylated Siglec-F ligands are up-regulated upon OVA challenge. (A) Serial sections of frozen lung from WT OVA-sensitized and -challenged mice were stained with antibodies against MBP (left panel, reddish brown color is positive) or Siglec-F (right panel, blue color is positive). Only the inflamed lungs were positive, as shown. (B) Recombinant soluble Siglec-F-Fc was used to probe for Siglec-F ligands in the lungs from OVA-sensitized and -challenged (OVA) or OVA-sensitized and PBS-challenged (No OVA) mice. Positive staining appears a dark reddish-brown color. The arginine-mutated R114A Siglec-F-Fc was used as a negative control, as it is deficient in sialylated ligand binding. Results shown are typical of n = 4 for each group and representative of 2 experiments. (C) Higher-magnification photomicrograph of an OVA-sensitized and -challenged lung section, probed with Siglec-F-Fc. Bronchiolar cells of the lung epithelia (white arrowheads) and mononuclear cells in the lung parenchyma (black arrowheads) were positive for Siglec-F ligands. For panels A-C, a 10×/0.25 DPlan dry objective lens was used to visualize images, and an Olympus BH2 camera was used to capture them. (D) Surface area of the Siglec-F ligand–positive bronchiolar epithelia. Mouse lungs were immunostained with Siglec-F-Fc and the area of bronchial epithelial Siglec-F-Fc immunostaining was quantitated by image analysis, with results expressed in squared micrometer/micrometer length of the basement membrane of the bronchus. WT mice challenged with OVA had a significant increase in levels of Siglec-F-Fc epithelial immunostaining compared with control non–OVA-challenged WT mice. (E) Mouse lungs were immunostained with Siglec-F-Fc, and the number of positive peribronchial cells quantitated was by image analysis. WT mice challenged with OVA had a significant increase in the numbers of peribronchial Siglec-F-Fc–positive cells compared with control non–OVA-challenged WT mice. (D-E) ***P < .001. Each error bar represents the standard error of the mean (SEM).
Siglec-F and sialylated Siglec-F ligands are up-regulated upon OVA challenge. (A) Serial sections of frozen lung from WT OVA-sensitized and -challenged mice were stained with antibodies against MBP (left panel, reddish brown color is positive) or Siglec-F (right panel, blue color is positive). Only the inflamed lungs were positive, as shown. (B) Recombinant soluble Siglec-F-Fc was used to probe for Siglec-F ligands in the lungs from OVA-sensitized and -challenged (OVA) or OVA-sensitized and PBS-challenged (No OVA) mice. Positive staining appears a dark reddish-brown color. The arginine-mutated R114A Siglec-F-Fc was used as a negative control, as it is deficient in sialylated ligand binding. Results shown are typical of n = 4 for each group and representative of 2 experiments. (C) Higher-magnification photomicrograph of an OVA-sensitized and -challenged lung section, probed with Siglec-F-Fc. Bronchiolar cells of the lung epithelia (white arrowheads) and mononuclear cells in the lung parenchyma (black arrowheads) were positive for Siglec-F ligands. For panels A-C, a 10×/0.25 DPlan dry objective lens was used to visualize images, and an Olympus BH2 camera was used to capture them. (D) Surface area of the Siglec-F ligand–positive bronchiolar epithelia. Mouse lungs were immunostained with Siglec-F-Fc and the area of bronchial epithelial Siglec-F-Fc immunostaining was quantitated by image analysis, with results expressed in squared micrometer/micrometer length of the basement membrane of the bronchus. WT mice challenged with OVA had a significant increase in levels of Siglec-F-Fc epithelial immunostaining compared with control non–OVA-challenged WT mice. (E) Mouse lungs were immunostained with Siglec-F-Fc, and the number of positive peribronchial cells quantitated was by image analysis. WT mice challenged with OVA had a significant increase in the numbers of peribronchial Siglec-F-Fc–positive cells compared with control non–OVA-challenged WT mice. (D-E) ***P < .001. Each error bar represents the standard error of the mean (SEM).
Sialic acid–dependent Siglec-F ligands are constitutively present in bronchial epithelia, and up-regulated upon OVA challenge
Exploring the possible role of Siglec-F interactions with its ligands in normal and allergic conditions, we also looked for Siglec-F ligands in the lung by probing tissue sections with Siglec-F-Fc, a recombinant soluble protein containing the extracellular domain of Siglec-F and the Fc region of human IgG (Figure 2B-C).18 In nonchallenged mice, staining was detected only along the lining of the bronchial epithelium. Much reduced staining was observed with a mutant probe (R114A Siglec-F-Fc) deficient in Sia binding, confirming that binding of Siglec-F-Fc is indeed primarily Sia dependent (Figure 2B). Of interest, ligands were detected after OVA challenge not only on the bronchial epithelia (where it was increased in extent and amount; Figure 2D), but also throughout the inflamed peribronchial area, which included mononuclear leukocytes (Figure 2E). No such up-regulation of ligands was seen in non–OVA-sensitized mice given the intranasal antigen challenge only (data not shown). Previous studies have shown that antibody-mediated cross-linking of CD33-related Siglecs causes negative signaling,10–13 and by analogy a natural ligand-mediated cross-linking likely causes a similar type of negative signaling. Taken together with the up-regulation of Siglec-F on eosinophils upon OVA challenge, these data suggest that Siglec-F and its ligands might mediate a negative feedback loop controlling eosinophil-mediated allergic responses.
Siglec-F expression is inducible on T cells
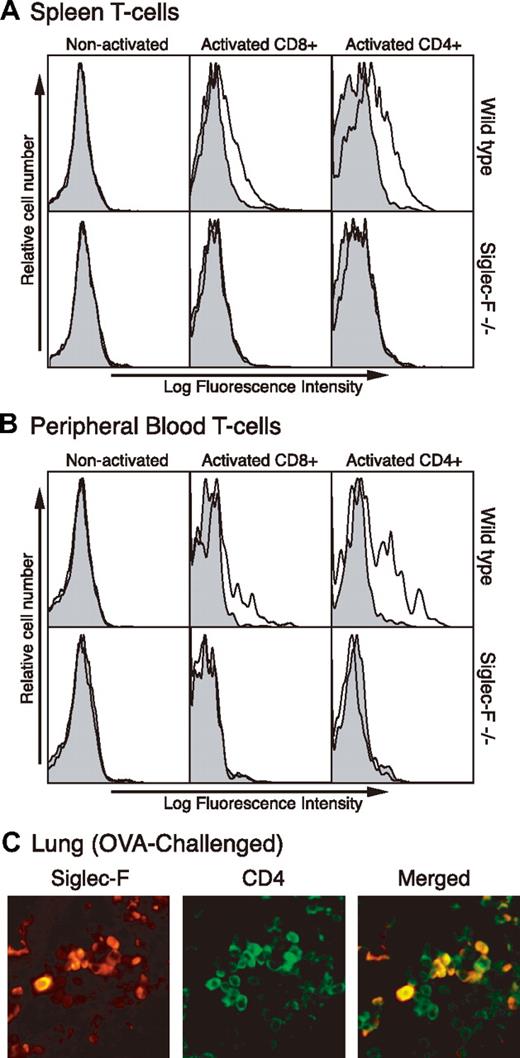
Th2 cells also play a crucial role in allergic conditions.25–27 Since mouse T cells are not known to express Siglecs, we tested if they express Siglec-F upon in vitro stimulation. Although nonactivated T cells did not express Siglec-F, both CD8+ and CD4+ T cells from spleen and peripheral blood showed expression upon activation. Cells from Siglec-F–null mice (“Generation of Siglec-F–deficient mice”) were used as a negative control, to confirm that all staining seen on the activated WT mouse cells was specific (Figure 3A-B).
Siglec-F expression is induced on activated mouse T cells in vitro and in vivo. (A) Spleen mononuclear leukocytes and (B) peripheral-blood cells were isolated and T cells activated in vitro by anti-CD3 and anti-CD28 for 3 days. Activated cells were stained by anti–Siglec-F (line) or control antibody (shaded) and analyzed by flow cytometry. Anti-CD4 or anti-CD8 was used to gate on subgroups of T cells. (C) Lung sections from chronically OVA-challenged WT mice were stained with anti-CD4 and anti–Siglec-F antibodies. Background staining with secondary antibody alone was minimal (data not shown). A 40×/0.65 DPlan dry objective lens was used to visualize images, and an Olympus BH2 camera was used to capture them.
Siglec-F expression is induced on activated mouse T cells in vitro and in vivo. (A) Spleen mononuclear leukocytes and (B) peripheral-blood cells were isolated and T cells activated in vitro by anti-CD3 and anti-CD28 for 3 days. Activated cells were stained by anti–Siglec-F (line) or control antibody (shaded) and analyzed by flow cytometry. Anti-CD4 or anti-CD8 was used to gate on subgroups of T cells. (C) Lung sections from chronically OVA-challenged WT mice were stained with anti-CD4 and anti–Siglec-F antibodies. Background staining with secondary antibody alone was minimal (data not shown). A 40×/0.65 DPlan dry objective lens was used to visualize images, and an Olympus BH2 camera was used to capture them.
We next asked if the same induction occurs on CD4+ T cells within the lung during an OVA-elicited allergic response. As there are very few peribronchial T cells present during an acute OVA challenge, we used samples from mice that were OVA challenged for a longer period of time—twice a week for one month, following regular acute challenge.34 Staining lung tissue sections from such mice with anti-CD4 and anti–Siglec-F antibodies showed some overlap of reactivity (Figure 3C), indicating induction of Siglec-F expression on some CD4+ T cells in vivo. Thus, induction of Siglec-F on CD4+ T cells could be a further component of the proposed negative feedback loop regulating allergic responses in this model, in combination with the up-regulation of Siglec-F ligands in the lung.
Generation of Siglec-F–deficient mice
Based on our findings, we hypothesized that lack of Siglec-F would allow an exaggerated eosinophilic response to OVA challenge. To test this hypothesis, we generated Siglec-F–null mice (hereafter called Siglec-F−/− mice) through homologous recombination and Cre-loxP–mediated excision of critical regions of the gene (Figure S1 and Document S1). While Siglec-F−/− mice have a similar number of eosinophils as the WT controls, they completely lack Siglec-F expression, as expected (Figure S1).
Siglec-F−/− mice were viable and fertile in a pathogen-free, limited-access barrier facility, with no obvious developmental or morphologic defects. No abnormalities were found in baseline total blood cell counts, platelet counts, and blood chemistries (data not shown). Leukocyte subgroup counts in lymphoid organs and serum immunoglobulin levels showed no changes, and the null mice were normal by histology studies (data not shown). No differences were found between WT and Siglec-F−/− mice in some immunologic assays, such as air pouch–lipopolysaccharide inflammation looking at neutrophil recruitment,35 group A streptococcus skin infection and wound healing assays evaluating lesion formation and bacteria killing,36 or oxazolone-ear painting to test contact hypersensitivity37 (data not shown). Overall, the Siglec-F–deficient mice are grossly normal in organ/tissue development and function, and in some innate immune responses not involving eosinophils.
Siglec-F−/− mice show enhanced lung eosinophilic inflammation, and peripheral blood and bone marrow eosinophilia, in a lung allergy model
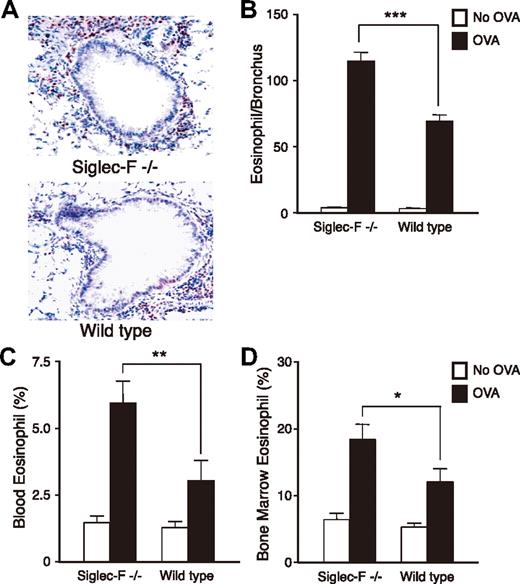
To test if Siglec-F plays a role in eosinophil-mediated disorders, we next compared Siglec-F−/− and WT mice in the lung responses to OVA challenge. OVA-challenged Siglec-F−/− mice exhibited more prominent peribronchial eosinophil infiltration than the WT controls (Figure 4A-B). We also enumerated eosinophils in Wright-Giemsa–stained bone marrow and peripheral-blood smears. Although baseline levels were similar, the OVA-challenged Siglec-F−/− mice had significantly more eosinophils in both blood and bone marrow (Figure 4C-D). In studying the bone marrow, we noticed increased metamyelocytic eosinophil precursors in OVA-challenged Siglec-F−/− mice (data not shown). Thus, although Siglec-F−/− mice have normal eosinophil levels in the baseline state, they manifest eosinophil overproduction upon OVA challenge. This is likely a major mechanism explaining the increased airway eosinophilia. There was no overall increase in numbers of infiltrating CD4+ cells in the lung tissues of the Siglec-F−/− mice compared with WT mice (data not shown).
Siglec-F−/− mice show elevated eosinophilic inflammation in lung, peripheral blood, and bone morrow in an OVA-induced lung allergy model. WT or Siglec-F−/− mice were either OVA sensitized and challenged (OVA) or OVA sensitized and PBS challenged (No OVA). All groups were compared for numbers of eosinophils in airway (A,B), blood (C), and bone marrow (D) (n = 6 mice/group, data shown is representative of 3 experiments). (A) WT and Siglec-F−/− OVA lung sections were stained for MBP. Dark red–stained peribronchial MBP+ cells were counted as eosinophils, and 8 to 10 bronchi/slide were counted. A 20×/0.40 DPlan dry objective lens was used to visualize images, and an Olympus BH2 camera was used to capture them. (B) Quantitative result derived from panel A, expressed as the number of eosinophils per bronchus. (C) Peripheral-blood leukocytes and (D) bone marrow cells were stained with Wright-Giemsa and differential cell counts taken under a light microscope. (B-D) *P < .05; **P < .01; ***P < .001. Each error bar represents the SEM.
Siglec-F−/− mice show elevated eosinophilic inflammation in lung, peripheral blood, and bone morrow in an OVA-induced lung allergy model. WT or Siglec-F−/− mice were either OVA sensitized and challenged (OVA) or OVA sensitized and PBS challenged (No OVA). All groups were compared for numbers of eosinophils in airway (A,B), blood (C), and bone marrow (D) (n = 6 mice/group, data shown is representative of 3 experiments). (A) WT and Siglec-F−/− OVA lung sections were stained for MBP. Dark red–stained peribronchial MBP+ cells were counted as eosinophils, and 8 to 10 bronchi/slide were counted. A 20×/0.40 DPlan dry objective lens was used to visualize images, and an Olympus BH2 camera was used to capture them. (B) Quantitative result derived from panel A, expressed as the number of eosinophils per bronchus. (C) Peripheral-blood leukocytes and (D) bone marrow cells were stained with Wright-Giemsa and differential cell counts taken under a light microscope. (B-D) *P < .05; **P < .01; ***P < .001. Each error bar represents the SEM.
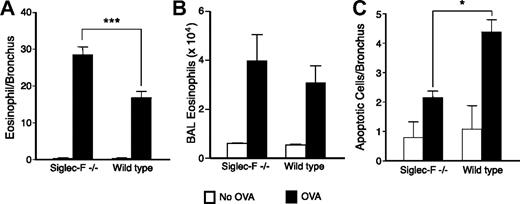
Eosinophil resolution is delayed, and apoptosis of peribronchial cells is impaired by Siglec-F deficiency
We next asked whether eosinophil clearance or emigration from the lung was affected by Siglec-F deficiency. Siglec-F−/− mice showed delayed eosinophil clearance from the lung (data from day 7 are in Figure 5A). There was no statistically significant change in BAL fluid eosinophil counts in the Siglec-F−/− mice compared with that in WT mice (Figure 5B), an observation that suggests the emigration of eosinophils may not be significantly affected. Human Siglec-8 can induce eosinophil apoptosis upon in vitro antibody cross-linking.12 Thus, we tested if Siglec-F contributes to eosinophil apoptosis. TUNEL staining on lung sections revealed diminished peribronchial-cell apoptosis in Siglec-F−/− mice (Figure 5C). These apoptotic cells included eosinophils (data not shown). A very small percentage of the MBP+ cells was apoptotic (likely because apoptotic cells are cleared rapidly), making quantitation of these small numbers unreliable. Regardless, diminished eosinophil apoptosis in Siglec-F−/− mice may further help explain the elevated peribronchial eosinophil accumulation and delayed eosinophil resolution.
Eosinophil resolution after OVA challenge is delayed in Siglec-F−/− mice and peribronchial-cell apoptosis is decreased. (A) Mice were killed 7 days after the last OVA challenge. Eosinophils/bronchus were enumerated, as in Figure 4. (B) Eosinophils in the BAL were counted, as described in “Materials and methods.” (C) Lung sections were stained for apoptotic cells by TUNEL assay (n = 4, data representative of 2 experiments). (A,C) *P < .05; ***P < .001.
Eosinophil resolution after OVA challenge is delayed in Siglec-F−/− mice and peribronchial-cell apoptosis is decreased. (A) Mice were killed 7 days after the last OVA challenge. Eosinophils/bronchus were enumerated, as in Figure 4. (B) Eosinophils in the BAL were counted, as described in “Materials and methods.” (C) Lung sections were stained for apoptotic cells by TUNEL assay (n = 4, data representative of 2 experiments). (A,C) *P < .05; ***P < .001.
Anti–Siglec-F antibody induces enhanced apoptosis in eosinophils from IL-5 transgenic mice in vitro
Since it is difficult to study the proposed cross-linking of Siglec-F by the up-regulated ligands in vitro, we instead examined the effects of an anti–Siglec-F monoclonal antibody. As it is difficult to obtain eosinophils from normal mice, we used cells from IL-5 transgenic mice. We found that while the antibody alone did not have any effect on the level of background apoptosis due to the withdrawal of IL-5, the addition of a secondary cross-linking antibody enhanced apoptosis (P < .001, Figure S2).
Effects of Siglec-F elimination on some other features of the asthmalike response in mice
The OVA-challenge model in mice also shows some other features of classical bronchial asthma. Mucus production was evaluated using the traditional periodic acid Schiff staining to detect mucus-producing goblet cells. Overall, no clear difference was detected between Siglec-F−/− and WT mice (data not shown). To verify this result, and to explore an improved method to study mucus production, we quantified mucin sialic acid content in BAL. Our new method takes advantage of the fact that mucins are resistant to proteinase digestion due to heavy O-glycosylation,32 allowing direct measurement of sialic acid content in the mucins. Using this method, we noted a trend toward increased mucin expression in Siglec-F−/− mice (1.24 ± 0.23 μM versus 1.76 ± 0.20 μM sialic acid), although this was not statistically significant (P = .09).
Some other typical features of classical asthma were also not obviously affected by Siglec-F deficiency under the conditions of this study. Total serum IgE levels were similar in OVA-challenged WT and Siglec-F−/− mice (data not shown). As mentioned earlier, there was no statistically significant change in BAL eosinophil number, and the number of peribronchial CD4+ cells was also unaffected by the Siglec-F deficiency. We also checked airway hyperresponsiveness to methacholine aerosol. Again, although we noticed a trend toward higher airway resistance in the Siglec-F−/− mice, there was no overall significant difference detected in either invasive or noninvasive measurements (data not shown). Regardless, our data with Siglec-F–null mice in this lung allergy model support the hypothesis that Siglec-F and its ligands are up-regulated as part of a negative feedback loop regulating eosinophilic and/or T-cell responses in allergy.
Discussion
We have provided here the first in vivo evidence for an inhibitory function of a CD33rSiglec. Expression of Siglec-F was up-regulated on eosinophils and induced on T cells during an induced lung allergic response. Also, Sia-dependent ligands for Siglec-F were expressed in the lung airways, and up-regulated during the allergic response. We hypothesized that the combination of increased Siglec-F and of its ligands serves as a negative feedback signal to eosinophils and T cells through Siglec-F. Indeed, studies in Siglec-F–null mice confirmed involvement of the molecule in regulating eosinophil numbers. In this regard, it is notable that the Siglec-F–null animals did not show any obvious eosinophil changes in their baseline state. We speculate that the effects of Siglec-F deficiency are manifest only in the presence of activating stimuli, possibly because of the altered cytokine environment that could affect the outcome of signaling via Siglec,13 or because of the lack of T-cell response via Siglec-F that is induced only upon stimulation.
The mechanism of regulation of bone marrow, blood, and lung eosinophil numbers by Siglec-F needs further study. The delayed eosinophil clearance from the lung in Siglec-F−/− mice may be partly due to diminished cell apoptosis. Apoptosis is an important mechanism to clear accumulated eosinophils and resolve airway eosinophilic inflammation, and correlates with the clinical severity of asthma.38 In vitro antibody cross-linking of Siglec-8 on isolated human eosinophils is known to cause apoptosis,12 and we also observed enhanced apoptosis of mouse eosinophils by antibody cross-linking of Siglec-F in vitro. By extending our observations, we propose that extensive cross-linking of Siglec-F by its ligands induces apoptosis of eosinophils under inflammatory conditions.
In addition to the possibility of direct induction of apoptosis or inhibition of marrow precursors and/or mature eosinophils by Siglec-F, this molecule could regulate eosinophil production and recruitment by modifying Th2-cell functions, which are known to play an essential role in allergic disorders such as asthma. Th2 cytokines such as IL-5 play an important role in bone marrow eosinophil production, as well as in preventing eosinophil apoptosis. Thus, Siglec-F–mediated inhibition of Th2-cell cytokine production in vivo might influence the number of eosinophils, independent of direct effects of Siglec-F on eosinophils. Indeed, our preliminary results indicate that the absence of Siglec-F enhances IL-5 production by OVA-stimulated T cells in vitro.
There are additional possible mechanisms for peribronchial eosinophilia in Siglec-F−/− mice. For example, eosinophil trafficking may be affected, and Siglec-F–ligand interactions may play a role in this process. Changes in eosinophil interactions with their ligands could further affect other aspects of eosinophil behavior, such as production and release of mediators and cytokines, or response to cytokines. Further studies to determine the cell type(s) that carry the ligand and the structure of the ligands could help in understanding how this interaction affects functions of eosinophils and T cells and/or the cell types carrying the ligand. Concerning the up-regulation of Siglec-F ligands in the lung parenchyma and airway epithelium, it is worth mentioning that sulfated sialyl-Lewis X structures occur in bronchial mucins.39,40 Furthermore, there is increasing evidence that inflammation affects glycosylation and sulfation of mucins. For example, TNF-α up-regulates human bronchial mucosal expression of α1-3 fucosyltransferases, α2-3 sialyltransferases, and sulfotransferases,40 the enzymes that would contribute to the synthesis of such sulfated, sialylated Lewis epitopes.
Mucus production was not significantly changed by Siglec-F deficiency. Since a variety of inflammatory mediators can stimulate mucus secretion, the lack of significant change is likely due to mediators produced from other cell types that do not express Siglec-F. Airway hyperresponsiveness was also not significantly affected by Siglec-F deficiency. In the mouse strain background used here, airway inflammation is more prominent than airway hyperresponsiveness.41
Finally, we should note that management of human asthma and several other eosinophil-related disorders has traditionally relied on symptomatic therapy and broadly acting agents such as corticosteroids, which can also have multiple side effects.42 The current work identifies one of the endogenous down-regulating mechanisms in such disorders. By extension of our finding, administration of synthetic ligands that cross-link Siglec-F may alleviate eosinophil-mediated disorders by a Siglec-F–dependent mechanism, such as augmentation of eosinophil clearance and/or inhibition of IL-5 production and release from Th2 cells. As discussed earlier, it is reasonable to speculate that the human isofunctional paralog Siglec-8 contributes similarly in human eosinophil-mediated disorders. Thus, we have defined a potential novel approach to the therapy of human asthma and other eosinophil-related diseases.
Authorship
Contribution: M.Z., T.A., J.Y.C., and M.M. performed the research; D.H.B. and A.V. designed the research; M.Z., T.A., D.H.B., and A.V. wrote the paper. M.Z. and T.A. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ajit Varki, UCSD, 9500 Gilman Dr, La Jolla, CA 92093-0687; e-mail: a1varki@ucsd.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by National Institutes of Health grants P01 HL57345 (A.V.), AI38425, and AI33977 (D.H.B.).
We thank Lisa Wiggleton, Melain Raguseo, Kirsti McElwain, and Shauna McElwain for excellent technical assistance, and Nissi Varki for expert advice on histologic analysis. We also thank Jennifer Stevenson and Sandra Diaz for critical reading of the paper.