Infantile osteopetrosis includes various genetic disorders of bone remodeling that lead to early death in the absence of stem cell transplantation. Johansson et al. have provided the first evidence in mice that gene therapy might eventually help.
In this issue of Blood, Johansson and colleagues have provided the first evidence that neonatal gene therapy targeted to hematopoietic stem cells can cure a murine model of osteopetrosis (the oc/oc mice). These findings follow the recent demonstrations by the same group of successful major histocompatibility complex (MHC)–matched neonatal hematopoietic stem cell transplantation (HSCT) in oc/oc mice,1 and by Frattini et al2 that in utero HSCT can reverse the oc/oc phenotype, even across the MHC barrier. Altogether, these findings illustrate the importance and the effectiveness of early transplantation of normal or gene-corrected hematopoietic stem cells to cure osteopetrosis. Infantile autosomal recessive (AR) osteopetroses include a heterogeneous group of genetic disorders of bone remodeling.3 Filling of the bone marrow cavity with new bone, ensuing in pancytopenia and extramedullary hematopoiesis; entrapment of cranial nerves, leading to visual and hearing impairment; and reduced growth are typical manifestations of AR osteopetrosis. If untreated, 75% of these patients die by 4 years of age, most often due to overwhelming infections and pancytopenia.
Genetic defects of the TCIRG1 gene, encoding for a subunit of the osteoclast proton pump essential for bone resorption, account for osteopetrosis in oc/oc mice and for 50% to 60% of all cases of AR osteopetrosis in humans. In this form, osteoclasts are present, but they are unable to mediate bone remodeling (see figure). HSCT represents the only available cure for severe AR osteopetrosis in humans, with over 70% disease-free survival when a human leukocyte antigen (HLA)–identical donor is available, but with far less satisfactory results when HSCT is performed from HLA-mismatched related donors. Furthermore, conservation of vision and growth is better if the transplantation is performed early in life.4 In this regard, demonstration that neonatal gene therapy can correct osteopetrosis in oc/oc mice is of the utmost importance. Johansson and colleagues have shown that fetal liver cells, transduced with a tcirg1-containing retroviral vector, can be induced to differentiate in vitro into bone-reabsorbing osteoclasts upon culture with macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-kappaB ligand (RANKL). In vivo injection of gene-corrected cells into sublethally irradiated oc/oc mice at day +1 of life resulted in prolonged survival and significant correction of the skeletal phenotype in a significant proportion of cases. However, a number of problems remain to be solved before a similar approach is proposed in humans.
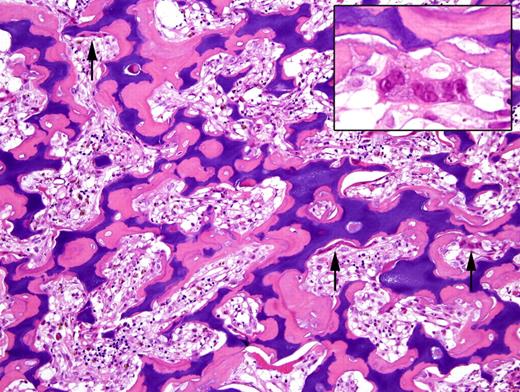
Photomicrograph of a bone biopsy from a patient with osteopetrosis, stained with haematoxylin-eosin. The amount of bone is markedly increased and represented by primary spongiosa (calcified cartilage rimmed by osteoid tissue). Note the numerous osteoclasts (arrows), laying along the trabeculae surface, without evidence of bone resorption (inset). Lacunae are obviously narrowed and the hematopoetic tissue reduced (courtesy of Professor F. Facchetti, Department of Pathology, University of Brescia, Italy).
Photomicrograph of a bone biopsy from a patient with osteopetrosis, stained with haematoxylin-eosin. The amount of bone is markedly increased and represented by primary spongiosa (calcified cartilage rimmed by osteoid tissue). Note the numerous osteoclasts (arrows), laying along the trabeculae surface, without evidence of bone resorption (inset). Lacunae are obviously narrowed and the hematopoetic tissue reduced (courtesy of Professor F. Facchetti, Department of Pathology, University of Brescia, Italy).
Final confirmation that gene therapy in oc/oc mice is able to correct the phenotype permanently, and that this is due to targeting of stem cells, will require prolonged observation and serial transplantation procedures. Introduction of tcirg1-specific regulatory elements is likely needed to maintain a physiological response to signals that govern bone resorption. Moreover, the large number of stem cells that are injected to correct oc/oc mice, the need for myeloablation, and the timing (day +1 of life) required for the procedure to be effective are formidable obstacles that need to be addressed in humans.
TCIRG1 defects only account for about half of the cases of AR osteopetrosis in humans. Other forms (such as those due to OSTM1 and CLCN7 gene defects) carry the burden of intrinsic retinal and neuronal degeneration, which are not corrected by HSCT. This indicates that molecular diagnosis and careful discussion of the potential benefits and limits of neonatal HSCT and/or gene therapy should all take place soon after birth to make the remarkable achievements of Johansson and colleagues applicable in the human situation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■