Abstract
Tumor-infiltrating myeloid cells, including tumor-associated macrophages (TAMs), have been implicated in tumor progression. We recently described a lineage of mouse monocytes characterized by expression of the Tie2 angiopoietin receptor and required for the vascularization and growth of several tumor models. Here, we report that TIE2 expression in human blood identifies a subset of monocytes distinct from classical inflammatory monocytes and comprised within the less abundant “resident” population. These TIE2-expressing monocytes (TEMs) accounted for 2% to 7% of blood mononuclear cells in healthy donors and were distinct from rare circulating endothelial cells and progenitors. In human cancer patients, TEMs were observed in the blood and, intriguingly, within the tumors, where they represented the main monocyte population distinct from TAMs. Conversely, TEMs were hardly detected in nonneoplastic tissues. In vitro, TEMs migrated toward angiopoietin-2, a TIE2 ligand released by activated endothelial cells and angiogenic vessels, suggesting a homing mechanism for TEMs to tumors. Purified human TEMs, but not TEM-depleted monocytes, markedly promoted angiogenesis in xenotransplanted human tumors, suggesting a potentially critical role of TEMs in human cancer progression. Human TEMs may provide a novel, biologically relevant marker of angiogenesis and represent a previously unrecognized target of cancer therapy.
Introduction
Hematopoietic cells of diverse lineages contribute to tumor progression.1–7 Among these cells, tumor-associated macrophages (TAMs) play important roles in tumorigenesis.3,8–10 TAMs derive from circulating monocytes, which differentiate into macrophages upon homing to tumors. In tumors, TAMs play dichotomous functions. Although TAMs may exert direct antitumor activities,11,12 increasing data indicate that they are skewed by the tumor microenvironment to a protumoral phenotype.8,13 Indeed, TAMs can blunt antitumor immunity and stimulate angiogenesis, cell migration, invasion, and metastasis.3,9 Whereas the divergent TAM functions (ie, antitumoral and protumoral activities) are thought to be contextually modulated by the tumor microenvironment,8 emerging data suggest that distinct subsets of circulating monocytes exist that are committed to specific functions, including tissue remodeling and proangiogenic activity.14–16 In this regard, we recently identified in mouse tumor models a subset of tumor-infiltrating monocytes characterized by the expression of the angiopoietin receptor Tie2/Tek,15,17 a molecule previously known to be restricted to endothelial and hematopoietic stem cells.18,19 In mice, Tie2-expressing monocytes (TEMs) home to tumors, where they are required for angiogenesis. Indeed, selective elimination of TEMs by a suicide gene strategy prevented angiogenesis and induced tumor regression.15,17 In these tumor models, angiogenesis was inhibited despite the fact that TAM recruitment to tumors was not impaired, indicating that a specific subset of myeloid-lineage cells was primarily responsible for promoting angiogenesis. This concept has received support from other studies that reported proangiogenic activity of selected myeloid cell subsets in mouse tumors, including VEGFR-1+CD11b+ myeloid cells, Gr-1+CD11b+ myeloid suppressor cells, and CD11c+MHC-II+ dendritic cell precursors.20–26 However, because most of the cell-surface markers used to identify these cell subsets are broadly expressed among myeloid cells, it is unclear whether these cell populations represent distinct rather than overlapping proangiogenic myeloid cells, possibly comprising the TEMs.7 Inhibiting the activities of proangiogenic myeloid cells should represent a valuable anticancer strategy. Yet, the rationale for this approach would obtain major support from the identification and functional characterization of proangiogenic myeloid cells in humans.
Here, we report that TIE2 expression in human peripheral blood (PB) identifies a novel subset of monocytes endowed with marked proangiogenic activity. These cells are distinct from classic inflammatory monocytes, express functional TIE2 receptor, and directly respond to angiopoietin-2 (Ang-2), a TIE2 ligand up-regulated in activated and angiogenic blood vessels. Interestingly, human TEMs are preferentially recruited to tumors, where they constitute the pre-eminent monocyte population distinct from TAMs.
Our results suggest a potential critical role of TEMs in human cancer angiogenesis and progression. These cells may provide a biologically relevant readout to monitor angiogenesis and may represent previously unrecognized targets of anticancer therapies.
Materials and methods
Cell purification and cell sorting
PB was obtained from healthy volunteers following informed consent, according to the Declaration of Helsinki and a protocol approved by the H. San Raffaele Bioethical Committee. Total leukocytes were analyzed after lysis of erythrocytes using ammonium chloride. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque gradient. Granulocytes and T and B lymphocytes were positively selected by magnetic sorting (using CD15, CD3, or CD19 MicroBeads, respectively; Miltenyi, Bergisch Gladbach, Germany). T-cell–depleted PBMCs were negatively selected by CD3 MicroBeads. Resident monocytes (CD16+CD14low) were enriched from PBMCs by negative selection of T, B, and natural killer (NK) cells (using a cocktail of CD3, CD19, and CD56 MicroBeads), followed by positive selection by CD16 MicroBeads. Inflammatory monocytes (CD16−CD14+) were enriched by negative selection of CD16+ cells, followed by positive selection by CD14 MicroBeads. For cell sorting, we used a Becton Dickinson (Heidelberg, Germany) FACS Vantage SE-FACSDiVa equipped with Argon Ion and HeNe lasers and the Quadra-Sort option. Sorted populations included CD14+TIE2+, CD14+TIE2−, and CD14+ monocytes (using FITC-conjugated anti-CD14 and PE-conjugated anti-TIE2 antibodies) and CD14+CD16+ and CD14+CD16− monocytes (using FITC-conjugated anti-CD14, PC5-conjugated anti-CD16, and PE-conjugated anti-TIE2 antibodies).
Flow cytometry and antibodies
Blood cells and tumors reduced to single-cell suspensions by collagenase digestion15 were processed for flow cytometry as follows. Cells were blocked in 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) containing the FcR Blocking Reagent (Miltenyi). After blocking, the Fc-blocked cells (∼106 cells) were stained with the following monoclonal antibodies (1-5 μg/mL): PE-conjugated anti-TIE2 (clone 83715), IgG1 isotypic control (clone IC002P), and anti-CCR2 from R&D Systems (Minneapolis, MN); PE-conjugated anti-CD19, anti-CD4, and IgG1 isotypic control (clone 11711) from BD Pharmingen (Heidelberg, Germany); PE-conjugated anti-CD133 (clone AC133) from Miltenyi; PE-conjugated anti-CD146 (clone S-endo1) from Biocytex (Marseille, France); FITC-conjugated anti–M-CSFR from R&D Systems; FITC-conjugated anti-CD62L, anti-CD31, anti-CD16, anti-CD14 from BD Pharmingen; FITC-conjugated anti-CD11c from Caltag (Burlingame, CA); FITC-conjugated anti-CD33 from ImmunoTools (Friesoythe, Germany); FITC-conjugated anti-CD13 from eBioscience (San Diego, CA); APC-conjugated anti-TIE2 (clone 83715) and anti–VEGFR-2 (clone 89106) from R&D Systems; APC-conjugated anti-CD45, anti-CD14, anti-CD11b, anti-CD3 from BD Pharmingen; PE-Cy7–conjugated anti-CCR5 from BD Pharmingen; PC5-conjugated anti-CD56 and anti-CD16 from Beckman Coulter (Hialeah, FL). All samples were analyzed by a FC500 flow cytometer (Coulter). Frequency of marker-positive cells is expressed as mean ± standard deviation (SD).
Real-time PCR
Total RNA was extracted from 1 × 105 to 5 × 105 cells using the RNeasy Micro kit (Qiagen, Hilden, Germany) and retrotranscribed using the superscript III First-Strand kit (Invitrogen, Carlsbad, CA). Taqman analysis of TIE2, VEGFR2, and GAPDH was performed on RNAse-treated cDNA using premade Taqman Gene Expression Assays from Applied Biosystems (Foster City, CA). Analyses were performed in 3 technical replicates, for 40 cycles in standard mode using an ABI7900HT apparatus. The SDS 2.2.1 software (Applied Biosystems) was used to analyze the data. The difference between the threshold cycle (Ct) of the TIE2 or VEGFR2 transcript and that of the endogenous control GAPDH (ΔCt) was used to determine gene expression. The average Ct of GAPDH was approximately 16 to 18 in both hematopoietic cells and human umbilical vein endothelial cells (HUVECs). ΔCt values are expressed as mean ± standard error. To obtain relative quantification values, we calculated the fold-change of each target mRNA over its content in a cell population taken as reference from the difference between the ΔCt of the target mRNA in the population of interest and the ΔCt of the target mRNA in the reference population (ΔΔCt) by the formula 2−ΔΔCt. For each relative value, an interval of confidence (P = .05) was calculated by the SDS 2.1.1 software; confidence intervals that did not overlap indicated statistically significant differences (P < .05).
Western-blot analysis
Fluorescence-activated cell sorter (FACS)–sorted cells (106) were lysed in Laemmli buffer, analyzed by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred on nitrocellulose, incubated for 2 hours with rabbit anti-TIE2 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) or mouse anti-β actin (1:5000; Sigma-Aldrich, St Louis, MO) antibodies, and revealed by goat anti–rabbit or antimouse HRP-conjugated antibodies (Upstate Biotechnology, Lake Placid, NY), followed by ECL plus (Amersham Bioscience, Freiburg, Germany) reaction and film exposures. For TIE2 immunoprecipitation, 107 cells were lysed with RIPA lysis buffer and incubated overnight with anti-TIE2 antibodies (Santa Cruz Biotechnology) and protein G microbeads (Miltenyi). Immunoprecipitated proteins were purified on separation columns (Miltenyi). Blots were incubated for 2 hours with a mouse monoclonal HRP-conjugated antiphosphotyrosine antibody (1:1000; Upstate Biotechnology).
Immunohistochemistry, immunofluorescence, and confocal analysis
Tissue specimens were obtained from surgical resections following informed consent according to the Declaration of Helsinki and the H. San Raffaele Bioethical Committee. Samples were embedded in OCT compound and snap-frozen. Five-micrometer sections were fixed in 4% paraformaldehyde for 15 minutes and immunostained. Briefly, sections were incubated with anti-TIE2 antibodies followed by detection with a polymeric labeling 2-step method (Super sensitive ihc detection system; Biogenex, San Ramon, CA) using 3,3′-diaminobenzidine as chromogen. After screening a panel of commercially available anti-TIE2 antibodies, 2 monoclonals, clone AB33 (Upstate Biotechnology; 1:200 dilution) and clone TEK9 (Reliatech, Braunschweig, Germany; 1:100 dilution), were chosen based on their specific and efficient staining of blood vessels and used with similar results. After immunostaining, the sections were counterstained with hematoxylin and eosin.
For immunofluorescence staining, frozen sections were blocked with 1% BSA and 5% fetal bovine serum (FBS). Sections were then stained with the following antibodies: goat polyclonal anti-TIE2 (R&D Systems) and monoclonal anti-TIE2 (clone AB33; Upstate Biotechnology) antibodies followed by donkey anti–goat or goat anti–mouse AlexaFluor 546–conjugated antibodies (Molecular Probes, Eugene, OR), respectively. To stain ECs and hematopoietic cells, the following antibodies were used: rabbit polyclonal anti–von Willebrand factor (DAKO, Carpinteria, CA) followed by AlexaFluor 488–conjugated antirabbit antibodies (Molecular Probes); FITC-conjugated anti-CD31, anti-CD13, anti-CD16, anti-CD14 monoclonal antibodies; or APC-conjugated anti-CD45, anti-CD34, anti-CD11b, anti-CD14 monoclonal antibodies (all from BD Pharmingen). Immunohistochemistry images (Figure 5) were taken using an Axioskop 2 plus direct microscope (Zeiss, Oberkochen, Germany). Images were captured by using the AxioCam HRc system and Axiovision 3.1 version 4.4 software (Zeiss). Confocal microscopy (Figure 6) used an Axioskop 2 plus direct microscope (Zeiss) equipped with a Radiance 2100 three-laser confocal device (BioRad, Segrate, Italy). Fluorescent signals from the individual fluorophores were sequentially acquired from single optical sections and analyzed by Paint Shop Pro X (Corel, Ottawa, Canada). Axioskop 2 microscope used Zeiss W-PI 10×/0.23 or Zeiss Plan-Neofluor 20×/0.5 numerical aperture objective lens.
Migration assays
Migration assays were performed in a 24-well transwell containing 8-μm pore-size inserts (Corning, Corning, NY) coated with Basement Membrane Extract Cultrex (Trevigen, Gaithersburg, MD). Chemoattractants were placed in serum-free DMEM medium (600 μL) in the bottom compartment of the chamber, and 100 μL of cell suspension (106 cells/mL) was added to the top compartment. The chambers were incubated at 37°C in humidified air with 5% CO2 for 12 hours. Migrated cells were labeled with 5 μg/mL calcein-am (Molecular Probes) in DMEM at 37°C for 1 hour and counted under a fluorescence microscope. Results are expressed as mean ± SD from 3 technical replicates. Number of cells migrated in the absence of chemoattractant (ie, medium) was used as a reference value and set to 100%. Ten percent FBS was used as positive control. Neutralizing anti-TIE2 antibodies (R&D Systems) were preincubated with cells for 20 minutes at 37°C. Purified goat anti–human IgGs were from Caltag. Ang-2 (R&D Systems) was heat inactivated for 30 minutes at 95°C.
In vivo tumor angiogenesis assays
FACS-sorted cells were coinjected together with U87 human glioma cells in 2 ratios: 1:20 (2.5 × 105 sorted cells together with 5 × 106 tumor cells) and 1:100 (5 × 104 sorted cells together with 5 × 106 tumor cells), subcutaneously in nude mice, and tumors were grown for 5 (1:20 ratio experiment) or 7 (1:100 ratio experiment) days. To quantify angiogenesis, serial sections spanning the whole tumor were cut for each 1 of 3 tumors per group and immunostained for CD31 (rat anti–mouse CD31 [BD Pharmingen], followed by goat anti–rat AlexaFluor 546–conjugated antibodies [Molecular Probes]). The total tumor area in every fifth section was scanned at × 100 magnification by a confocal microscope. We then measured the vascular area on individual confocal planes by computer-assisted digital image analysis.27 Counts were averaged to obtain the vascular area, and values are expressed as mean ± SD. Statistical significance was calculated by Student t test.
Results
TIE2 expression in human peripheral blood identifies a subset of CD14+ monocytes
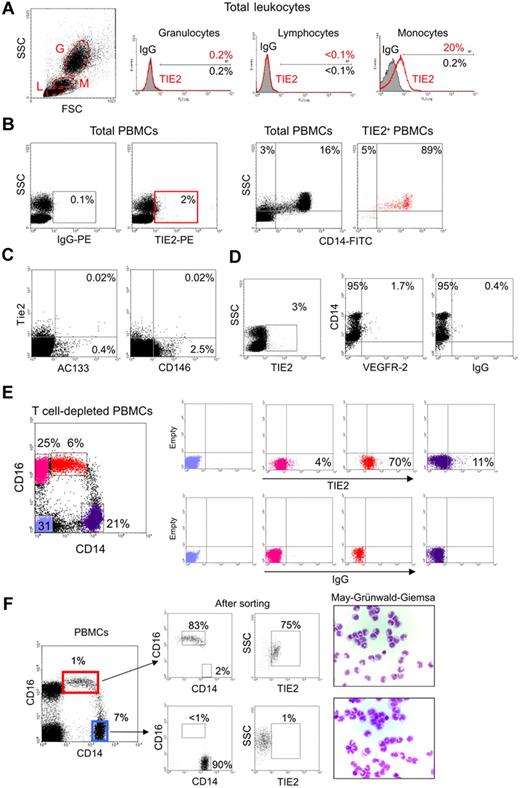
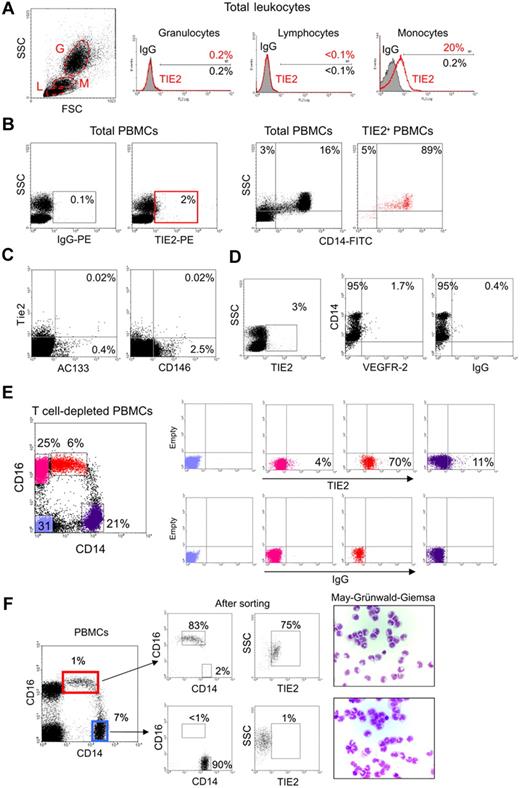
In order to investigate TIE2 expression by human hematopoietic cells, we stained peripheral blood (PB) leukocytes obtained from healthy donors with a mouse anti–human TIE2 monoclonal antibody (clone 83715; R&D Systems) and analyzed the cells by flow cytometry. A significant fraction of monocytes, but not granulocytes or lymphocytes identified on the basis of physical gating, were TIE2+ (Figure 1A). TIE2+ cells accounted for 1.6% to 7.4% (mean: 3.3% ± 1.5%; n = 16) of the total PB mononuclear cells (PBMCs) and expressed CD14 (Figure 1B), which is a component of the lipopolysaccharide receptor broadly expressed by human monocytes.16
A subset of human monocytes express the TIE2 angiopoietin receptor. (A) Flow cytometry analysis of PB granulocytes (G), lymphocytes (L), and monocytes (M) identified on the basis of physical gating (dot plot on the left; gates indicated by dashed line) shows TIE2 expression in a subset of monocytes (open red line in the histogram plots on the right; filled line IgG isotype control). Percentages of marker-positive cells are indicated. (B) The TIE2+ PBMCs (red gate in left panels) are mostly CD14+ monocytes (dot plots on the right). Representative analysis of at least 16 performed on different donors. (C) The vast majority of TIE2+ PBMCs do not express the CEC/EPC markers AC133 or CD146. Rare TIE2+AC133+ and TIE2+CD146+ cells may represent EPCs and CECs, respectively. Similar findings were obtained on 2 different PBMC samples. (D) A small subset of TIE2+ cells are VEGFR-2+CD14+, likely representing previously described monocytes with endothelial-like functional capacity. (E) T-cell–depleted PBMCs were stained with FITC-conjugated anti-CD14, PC5-conjugated anti-CD16, and PE-conjugated anti-TIE2 or isotype control antibodies. Expression of CD14 and CD16 (dot plot on the left) identifies 2 distinct monocyte subsets (see text). The gated cell populations (stained in different colors) were analyzed for expression of TIE2 (top right dot plots) versus isotype control (bottom right dot plots). Note that the CD14lowCD16+ fraction (resident monocytes; red dots) is highly enriched in TIE2+ cells, whereas the CD14highCD16− fraction (inflammatory monocytes; violet dots) contains few TIE2+ cells. CD14− cells (blue and pink dots) mainly represent B and natural killer cells and are mostly TIE2−. Representative analysis of at least 6 experiments performed on different donors. (F) The 2 main monocyte subsets were sorted from PBMCs using the indicated gates (dot plot on the left) and reanalyzed for expression of CD14, CD16, and TIE2 (dot plots on the right) and after cytospin and May-Grünwald-Giemsa staining.
A subset of human monocytes express the TIE2 angiopoietin receptor. (A) Flow cytometry analysis of PB granulocytes (G), lymphocytes (L), and monocytes (M) identified on the basis of physical gating (dot plot on the left; gates indicated by dashed line) shows TIE2 expression in a subset of monocytes (open red line in the histogram plots on the right; filled line IgG isotype control). Percentages of marker-positive cells are indicated. (B) The TIE2+ PBMCs (red gate in left panels) are mostly CD14+ monocytes (dot plots on the right). Representative analysis of at least 16 performed on different donors. (C) The vast majority of TIE2+ PBMCs do not express the CEC/EPC markers AC133 or CD146. Rare TIE2+AC133+ and TIE2+CD146+ cells may represent EPCs and CECs, respectively. Similar findings were obtained on 2 different PBMC samples. (D) A small subset of TIE2+ cells are VEGFR-2+CD14+, likely representing previously described monocytes with endothelial-like functional capacity. (E) T-cell–depleted PBMCs were stained with FITC-conjugated anti-CD14, PC5-conjugated anti-CD16, and PE-conjugated anti-TIE2 or isotype control antibodies. Expression of CD14 and CD16 (dot plot on the left) identifies 2 distinct monocyte subsets (see text). The gated cell populations (stained in different colors) were analyzed for expression of TIE2 (top right dot plots) versus isotype control (bottom right dot plots). Note that the CD14lowCD16+ fraction (resident monocytes; red dots) is highly enriched in TIE2+ cells, whereas the CD14highCD16− fraction (inflammatory monocytes; violet dots) contains few TIE2+ cells. CD14− cells (blue and pink dots) mainly represent B and natural killer cells and are mostly TIE2−. Representative analysis of at least 6 experiments performed on different donors. (F) The 2 main monocyte subsets were sorted from PBMCs using the indicated gates (dot plot on the left) and reanalyzed for expression of CD14, CD16, and TIE2 (dot plots on the right) and after cytospin and May-Grünwald-Giemsa staining.
Circulating endothelial cells (CECs) and endothelial progenitor cells (EPCs) can be detected at very low frequency in PB and are expected to express TIE2.28 The relatively high frequency of the TIE2+ cells and the fact that these cells express monocyte markers (see paragraph below) would, in principle, be sufficient to exclude that they represent CECs/EPCs. However, to formally rule out this possibility, we stained PBMCs with monoclonal antibodies directed against VEGFR-2, AC133, and CD146, which have been previously used to identify CECs/EPCs.28–30 We found that the vast majority of the PB TIE2+ cells were AC133−, CD146− (Figure 1C), and VEGFR-2− (Figure 1D), further indicating that they were distinct from circulating CECs/EPCs. We noted, however, that a small fraction (1%-2%) of the TIE2+ cells were CD14+VEGFR-2+, a phenotype previously associated with monocytes endowed with endothelial-like differentiation capacity.31
Recent studies have suggested that human CD14+ monocytes can be divided into 2 main subsets according to the expression of CD16, a Fc gamma receptor III.14 CD14highCD16− cells are the most abundant monocytes in PB and are thought to represent classical monocytes that mediate inflammatory responses (“inflammatory” monocytes), whereas CD14lowCD16+ cells are a less-characterized subset that are thought to represent the precursors of tissue-resident macrophages and are referred to as “resident” monocytes.14,16 Interestingly, we found that TIE2+ cells were comprised within CD14lowCD16+ monocytes and were mostly excluded from the CD14highCD16− subset (Figure 1E). In different samples (n = 10), analyzed either by multi-color flow cytometry (Figure 1E) or after FACS sorting of the 2 monocyte subsets (Figure 1F), TIE2+ cells accounted for 35% to 75% of the CD14lowCD16+ monocytes. These results indicated that TIE2 expression in PB specifically identified a subset of CD16+ monocytes distinct from common inflammatory monocytes.
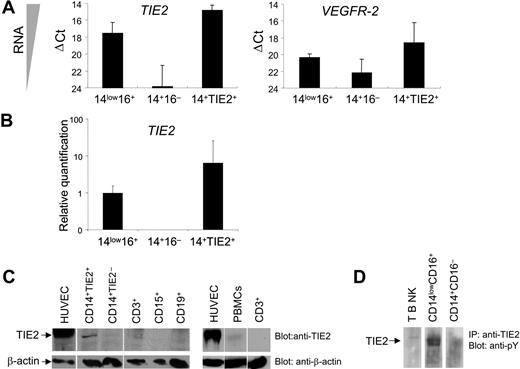
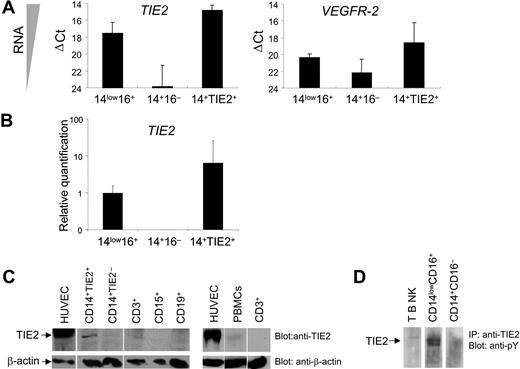
In order to verify TIE2 expression at the transcriptional level, we performed real-time PCR of TIE2 mRNA on FACS-sorted CD14+TIE2+ TEMs and on CD14lowCD16+ resident and CD14+CD16− inflammatory monocytes, using GAPDH as internal standard (Figure 2A-B). The TIE2 transcript was clearly expressed in CD14lowCD16+ resident but nearly undetectable in CD14+CD16− inflammatory monocytes, and it was significantly enriched (8-fold) in CD14+TIE2+ TEMs compared with the resident monocytes. However, TIE2 mRNA expression in TEMs was much lower than in human endothelial cells (HUVECs), used as positive control (ΔCt = 6.1 ± 0.6; n = 2). Of note, the VEGFR2 transcript, which was expressed in HUVECs to a similar level as TIE2 (ΔCt = 5.8), was expressed to a much lower level than TIE2 in resident monocytes and TEMs. These results argue against the possibility that low-level contamination of the TIE2+ cell fraction by CECs/EPCs was responsible for the recovery of the TIE2 signal from monocytes.
TIE2 receptor expression by TIE2+ monocytes. (A) TaqMan analyses of TIE2 and VEGFR2 transcripts in FACS-sorted monocyte subsets showing ΔCt values over endogenous control GAPDH. The lower the ΔCt, the higher the expression of the transcript in the target cell population. ΔCt values are expressed as mean ± standard error. Note that TIE2 transcript is clearly expressed in CD14lowCD16+ (14low16+) resident but nearly undetectable in CD14+CD16− (14 +16−) inflammatory monocytes. (B) Relative quantification values of TIE2 transcript in FACS-sorted monocyte subsets. TIE2 transcript is significantly enriched in CD14+TIE2+ (14+TIE2+) TEMs compared with the resident monocytes. For each relative value, an interval of confidence was calculated; confidence intervals that do not overlap indicate statistically significant differences (P < .05). (C) Western-blot analysis of TIE2 protein expression in the indicated cell populations. Blots were probed with C-terminus specific anti-TIE2 rabbit (top panels) or mouse anti-β actin (bottom panels) antibodies. The expected migration of each protein relative to molecular weight standards is indicated. Representative experiment of 3 performed. (D) TIE2 immunoprecipitated from CD14lowCD16+ resident monocytes is phosphorylated on tyrosine. Representative experiment of 2 performed.
TIE2 receptor expression by TIE2+ monocytes. (A) TaqMan analyses of TIE2 and VEGFR2 transcripts in FACS-sorted monocyte subsets showing ΔCt values over endogenous control GAPDH. The lower the ΔCt, the higher the expression of the transcript in the target cell population. ΔCt values are expressed as mean ± standard error. Note that TIE2 transcript is clearly expressed in CD14lowCD16+ (14low16+) resident but nearly undetectable in CD14+CD16− (14 +16−) inflammatory monocytes. (B) Relative quantification values of TIE2 transcript in FACS-sorted monocyte subsets. TIE2 transcript is significantly enriched in CD14+TIE2+ (14+TIE2+) TEMs compared with the resident monocytes. For each relative value, an interval of confidence was calculated; confidence intervals that do not overlap indicate statistically significant differences (P < .05). (C) Western-blot analysis of TIE2 protein expression in the indicated cell populations. Blots were probed with C-terminus specific anti-TIE2 rabbit (top panels) or mouse anti-β actin (bottom panels) antibodies. The expected migration of each protein relative to molecular weight standards is indicated. Representative experiment of 3 performed. (D) TIE2 immunoprecipitated from CD14lowCD16+ resident monocytes is phosphorylated on tyrosine. Representative experiment of 2 performed.
We then analyzed TIE2 receptor expression by Western-blot analysis of sorted hematopoietic populations using antibodies directed against the C-terminus of the TIE2 protein. As shown in Figure 2C, a band with the expected 145-kDa molecular weight of the TIE2 protein and comigrating with a major band in HUVECs was clearly detectable only in lysates of FACS-sorted CD14+TIE2+ cells and barely detectable in total PBMCs, among all blood cell subsets analyzed. TIE2 was auto-phosphorylated on tyrosine, as shown by immunoprecipitation from magnetically sorted resident monocytes and immunostaining with antiphosphotyrosine antibodies, indicating functional activation of the receptor in these cells (Figure 2D).
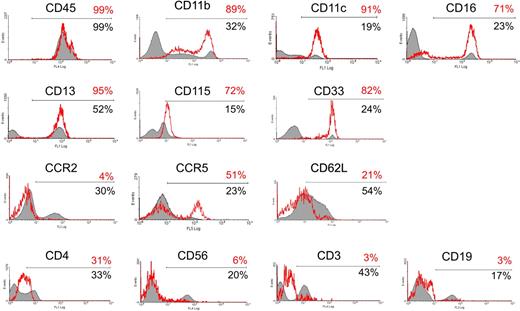
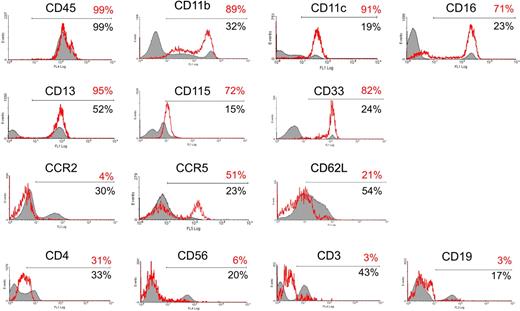
We further investigated the phenotype of circulating TIE2+ cells (Figure 3) and found that they were CD45+, CD11b (MAC-1)+, CD11c+, CCR2−, CCR5+, and L-selectin (CD62L)−, a surface profile that has been previously associated with resident monocytes.16 TIE2+ cells did not express the natural killer cell marker CD56 or, as expected, the lymphocyte-specific markers CD3 (T cells) and CD19 (B cells), whereas they expressed a low level of CD4, a T-lymphocyte coreceptor also expressed by monocytes. The TIE2+ cells homogeneously expressed CD33 (sialic-acid binding Ig-like lectin 3, a sialoadhesion integrin highly expressed by monocytes32 ), the macrophage-colony stimulating factor receptor (M-CSFR; also known as c-FMS or CD115, a chemokine receptor involved in the recruitment of monocytes to tumors33 ), and CD13 (aminopeptidase N, a myeloid marker34 ). Interestingly, expression of these markers has also been associated with an immature or progenitor cell phenotype.35
Characterization of the TIE2+ monocytes. (A) TIE2+ cells in unfractioned PBMCs were gated and analyzed for the expression of a panel of hematopoietic markers, as indicated in the histogram plots. The red open line shows expression of the indicated marker in the gated TIE2+ population (with the percentage of marker-positive cells in red); the filled line depicts expression of the indicated marker in the TIE2− cell population (with the percentage of marker-positive cells in black). The TIE2+ cells were CD45+, CD11b+, CD11c+, CD16+, CD33+, CD115+, and CD13+, which are all markers of monocytic cells. In addition, the TIE2+ cells were CCR2−, CD62L (L-selectin)−, and CCR5+, a surface profile previously associated with resident monocytes. As expected, the TIE2+ cells were CD56−, CD3−, and CD19− and thus distinct from natural killer cells and T and B lymphocytes. Representative analysis of 3 to 6 experiments performed on different donors.
Characterization of the TIE2+ monocytes. (A) TIE2+ cells in unfractioned PBMCs were gated and analyzed for the expression of a panel of hematopoietic markers, as indicated in the histogram plots. The red open line shows expression of the indicated marker in the gated TIE2+ population (with the percentage of marker-positive cells in red); the filled line depicts expression of the indicated marker in the TIE2− cell population (with the percentage of marker-positive cells in black). The TIE2+ cells were CD45+, CD11b+, CD11c+, CD16+, CD33+, CD115+, and CD13+, which are all markers of monocytic cells. In addition, the TIE2+ cells were CCR2−, CD62L (L-selectin)−, and CCR5+, a surface profile previously associated with resident monocytes. As expected, the TIE2+ cells were CD56−, CD3−, and CD19− and thus distinct from natural killer cells and T and B lymphocytes. Representative analysis of 3 to 6 experiments performed on different donors.
TIE2-expressing monocytes are recruited to human tumors
The analysis of blood samples obtained from 7 cancer patients at the time of surgery demonstrated in all cases the presence of TIE2+ monocytes with a surface phenotype similar to that of TEMs from healthy donors. In the patients, the frequency of TEMs ranged from 1.8% to 10.1% (mean: 4.9% ± 3.0%) of the total PBMCs. Further studies are required to assess whether TEM frequency in cancer patients significantly differs from the normal range and correlates with clinical parameters.
To study whether human TIE2+ monocytes are present in human solid tumors, we analyzed the hematopoietic infiltrate of 21 human cancer specimens, including kidney, colorectal, breast, gastric, pancreatic, and lung carcinomas, and soft tissue sarcomas, by 4-color flow cytometry analysis, immunohistochemistry, and confocal immunofluorescence microscopy (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
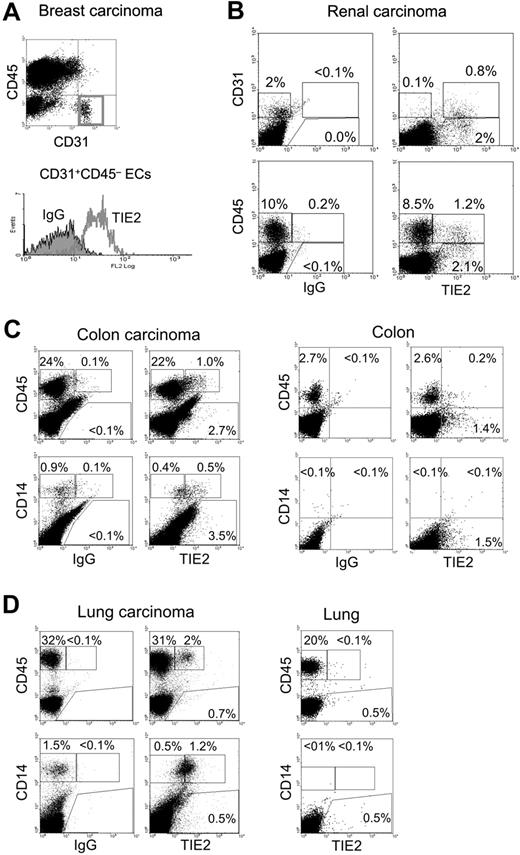
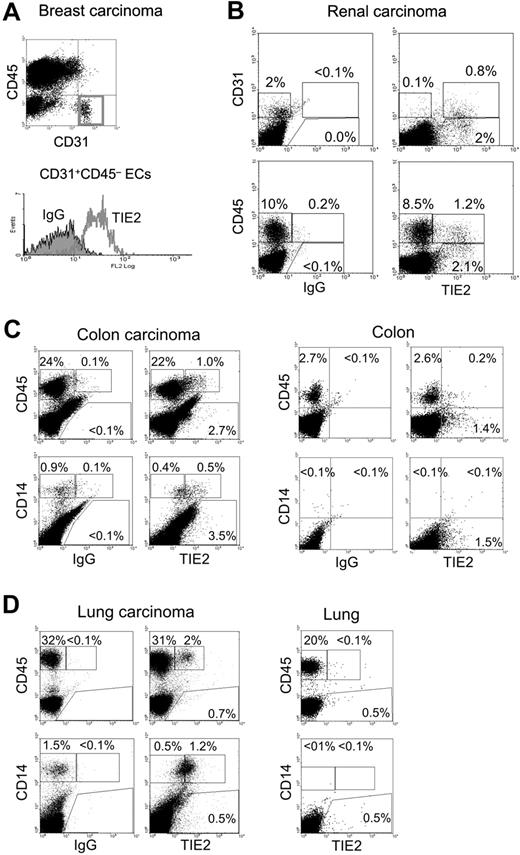
Fresh tumor tissues obtained from surgical resections were made into single-cell suspensions and analyzed by flow cytometry for the expression of (i) the pan-leukocyte marker CD45, (ii) the monocyte markers CD14 or CD11b, (iii) the EC markers CD31 or CD34, and (iv) TIE2 (Figure 4). Where possible, a sample of nonneoplastic tissue adjacent to the tumor was also obtained for comparative analysis. As expected, in all tumors analyzed (n = 9), the vast majority of ECs, defined as CD31+CD45− or CD34+CD45−, expressed TIE2 (Figure 4A-B). In addition to ECs, we noted that a small fraction (1%-12%) of the total CD45+CD31−/low tumor–derived leukocytes expressed TIE2 (Figure 4B). Interestingly, these TIE2+CD45+ cells were highly enriched in the CD14+ population (37%-72% TIE2+, n = 7), which is a small fraction of the hematopoietic infiltrate and likely represents monocytes or immature macrophages. Compared with the blood TEMs, the average expression level of TIE2 appeared substantially higher in the TIE2+ tumor-infiltrating monocytes. Two representative examples of these analyses are illustrated in Figure 4C-D, which shows that 4% and 6% of the CD45+ leukocytes and 55% and 70% of the CD14+ monocytes were TIE2+ in a colorectal (Figure 4C) and a lung (Figure 4D) carcinoma, respectively. Of note, in both tumors the wide majority of tumor-infiltrating CD45+ leukocytes, which may comprise TAMs, lymphocytes, and granulocytes, were TIE2−. Intriguingly, the frequency of TIE2+CD45+ cells was significantly lower or undetectable in nonneoplastic tissues adjacent to the tumors. As expected, normal tissues had a lower overall content of CD45+ hematopoietic cells than the tumors.
TIE2+ monocytes are found in human tumors. Flow cytometry analysis of the indicated tumor specimens, processed and analyzed as described in the text. FITC-conjugated anti-CD31 or anti-CD14, PE-conjugated anti-TIE2, or APC-conjugated anti-CD45 antibodies were used. (A) Breast carcinoma. Note that the gated CD31+CD45− tumor-derived ECs are TIE2+ (red open line in the histogram on the right; filled line is the IgG isotype control). (B) Renal carcinoma. In this tumor specimen, approximately 2% of the tumor-derived cells are CD31+ (CD45−, not depicted) ECs. The wide majority of these CD31+ tumor-derived ECs are TIE2+. Note that a fraction of the tumor-derived cells are CD31−TIE2+ non-ECs (top right dot plot). In the same tumor sample, approximately 10% of tumor-derived cells are CD45+ hematopoietic cells. Only a minor fraction (12%) of these CD45+ cells are TIE2+ (bottom right dot plot). (C) Colon carcinoma (left) and nonneoplastic colon mucosa (right). In the tumor, 4% of the abundant CD45+ hematopoietic cells and most of the CD14+ monocytes are TIE2+. In the normal mucosa, CD45+ hematopoietic cells are much less abundant than in the tumor, and only a few TIE2+ cells are found. Note that CD14+ monocytes are not detected in the normal mucosa. (D) Lung adenocarcinoma (left) and nonneoplastic lung tissue (right). In the tumor, more than 30% of the cells are CD45+ hematopoietic cells, of which 6% are TIE2+. Most of the tumor-derived CD14+ monocytes are TIE2+. In the normal lung tissue, the CD45+ hematopoietic cells are TIE2−; note that CD14+ monocytes are not found in this tissue.
TIE2+ monocytes are found in human tumors. Flow cytometry analysis of the indicated tumor specimens, processed and analyzed as described in the text. FITC-conjugated anti-CD31 or anti-CD14, PE-conjugated anti-TIE2, or APC-conjugated anti-CD45 antibodies were used. (A) Breast carcinoma. Note that the gated CD31+CD45− tumor-derived ECs are TIE2+ (red open line in the histogram on the right; filled line is the IgG isotype control). (B) Renal carcinoma. In this tumor specimen, approximately 2% of the tumor-derived cells are CD31+ (CD45−, not depicted) ECs. The wide majority of these CD31+ tumor-derived ECs are TIE2+. Note that a fraction of the tumor-derived cells are CD31−TIE2+ non-ECs (top right dot plot). In the same tumor sample, approximately 10% of tumor-derived cells are CD45+ hematopoietic cells. Only a minor fraction (12%) of these CD45+ cells are TIE2+ (bottom right dot plot). (C) Colon carcinoma (left) and nonneoplastic colon mucosa (right). In the tumor, 4% of the abundant CD45+ hematopoietic cells and most of the CD14+ monocytes are TIE2+. In the normal mucosa, CD45+ hematopoietic cells are much less abundant than in the tumor, and only a few TIE2+ cells are found. Note that CD14+ monocytes are not detected in the normal mucosa. (D) Lung adenocarcinoma (left) and nonneoplastic lung tissue (right). In the tumor, more than 30% of the cells are CD45+ hematopoietic cells, of which 6% are TIE2+. Most of the tumor-derived CD14+ monocytes are TIE2+. In the normal lung tissue, the CD45+ hematopoietic cells are TIE2−; note that CD14+ monocytes are not found in this tissue.
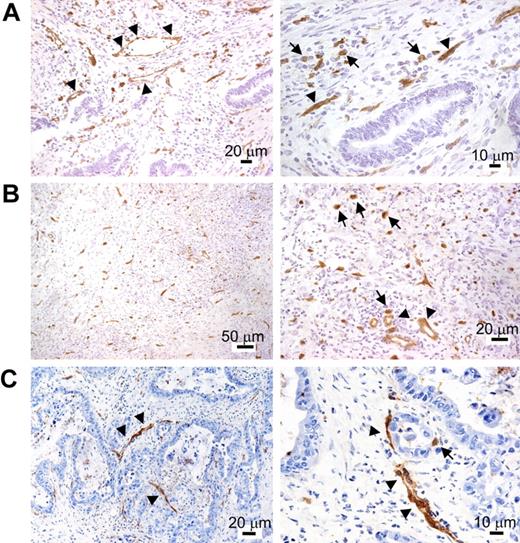
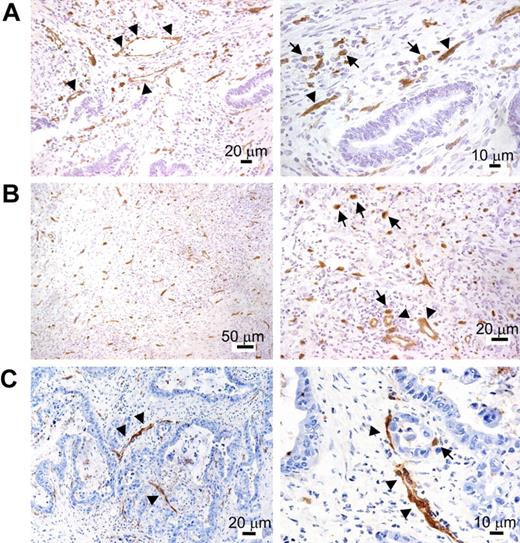
TIE2 immunostaining of cryostatic sections of cancer specimens (Table S1) showed, in addition to robust and near-uniform marking of blood vessels, the presence of scattered TIE2+ mononuclear cells within the tumor stroma (Figure 5). These cells appeared distinct from ECs because they did not show connection to blood vessels, had monocytic features (ie, roundish cytoplasmic outline and small nuclei), and showed uniform staining by anti-TIE2 antibodies lining the cell surface. Note that this type of single-marker analysis could not identify TIE2+ monocytes closely associated with TIE2+ blood vessels. Consistent with the flow-cytometry data, the frequency of the TIE2+ mononuclear cells was low albeit variable among different tumor specimens, with some tumors displaying minimal infiltration and others showing focal increase of TIE2+ cells. The vast majority of tumor-infiltrating hematopoietic cells, including TAMs, were TIE2−, ruling out Fc-dependent binding of the antibody to macrophages.
TIE2 immunohistochemistry of human cancer cryosections. After TIE2 immunostaining, the sections were counterstained with hematoxylin and eosin and shown at lower (left) or higher (right) magnification. (A) Colon adenocarcinoma. In addition to vascular ECs (arrowheads), TIE2 staining highlights the presence of stromal mononuclear elements morphologically consistent with monocytes (arrows). These cells appear inhomogeneously distributed, with foci of high density (arrows). (B) Gastric undifferentiated adenocarcinoma. Many TIE2+ mononuclear cells are found in the tumor stroma (arrows). Blood vessels are indicated by arrowheads. (C) Pancreatic adenocarcinoma. The majority of TIE2+ structures in the left panel are blood vessels (arrowheads). An individual TIE2+ mononuclear cell is shown (arrow) in the right panel.
TIE2 immunohistochemistry of human cancer cryosections. After TIE2 immunostaining, the sections were counterstained with hematoxylin and eosin and shown at lower (left) or higher (right) magnification. (A) Colon adenocarcinoma. In addition to vascular ECs (arrowheads), TIE2 staining highlights the presence of stromal mononuclear elements morphologically consistent with monocytes (arrows). These cells appear inhomogeneously distributed, with foci of high density (arrows). (B) Gastric undifferentiated adenocarcinoma. Many TIE2+ mononuclear cells are found in the tumor stroma (arrows). Blood vessels are indicated by arrowheads. (C) Pancreatic adenocarcinoma. The majority of TIE2+ structures in the left panel are blood vessels (arrowheads). An individual TIE2+ mononuclear cell is shown (arrow) in the right panel.
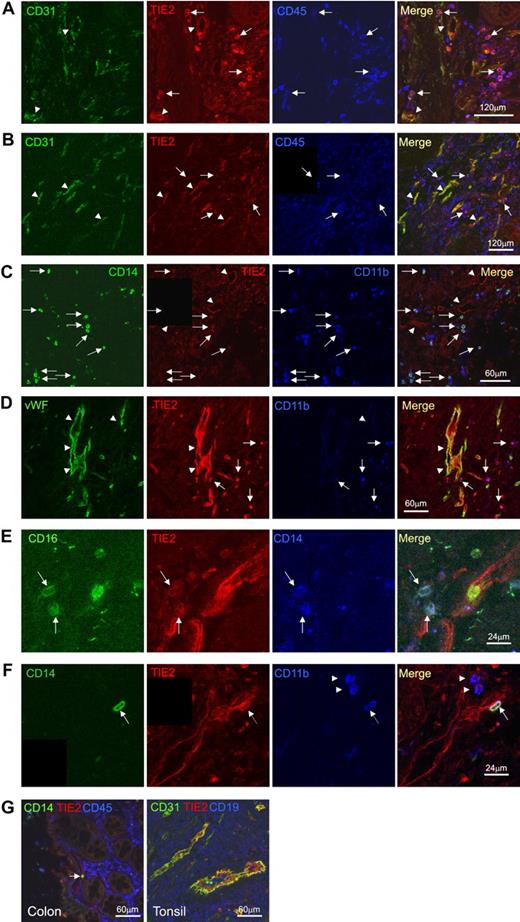
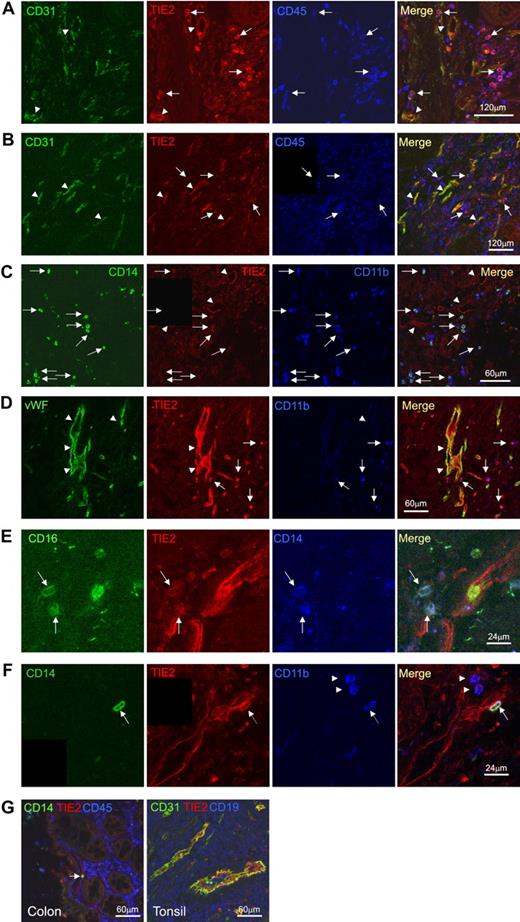
In order to confirm the hematopoietic, nonendothelial nature of the TIE2+ mononuclear cells, we performed triple immunofluorescence staining and confocal microscopy on selected frozen sections obtained from different tumor specimens (n = 8; Figure 6). Vascular ECs were clearly identified by their morphology, their organization in tubular structures, and the coexpression of TIE2 and CD31, CD34 (not shown), or von Willebrand factor (VWF). Scattered TIE2+ cells distinct from ECs (CD31− or CD34− or VWF−) were frequently observed that coexpressed the hematopoietic marker CD45. These cells had a small, rounded shape consistent with the morphology of monocytes and expressed the myeloid markers CD14, CD16, CD13 (not shown), and CD11b. These TIE2+ monocytes were often found in well-vascularized tumor regions and sometimes had a peri-endothelial location (Figure 6F). Whereas the majority of CD14+ monocytes were TIE2+, only a minority of the total CD45+ hematopoietic cells expressed TIE2, indicating that TIE2 was a distinguishing feature of tumor-infiltrating monocytes and was not expressed by the wide majority of TAMs.
Confocal immunofluorescence analysis of human cancer sections confirms the presence of TIE2+CD45+CD14+ tumor-infiltrating monocytes. (A) Colon adenocarcinoma analyzed for CD31 (green), TIE2 (red), and CD45 (blue). Confocal planes are shown individually and after merging. Several TIE2+CD45+CD31− hematopoietic cells (merge of red and blue giving purple; arrows) are found within the tumor stroma. Note that TIE2 is expressed by vascular ECs (merge of green and red giving yellow; arrowheads), which are TIE2+CD45−CD31+. (B) Gastric adenocarcinoma analyzed for CD31 (green), TIE2 (red), and CD45 (blue). Some TIE2+CD45+CD31− hematopoietic cells are found in the tumor stroma (arrows) together with TIE2+CD45−CD31+ tumor blood vessels (arrowhead). Scale bars as indicated. (C) Colon adenocarcinoma analyzed for CD14 (green), TIE2 (red), and CD11b (blue). Several TIE2+CD14+CD11b+ monocytes (arrows) are found within the tumor stroma. Note TIE2 expression by TIE2+CD14−CD11b− vascular ECs (arrowheads). (D) Gastric adenocarcinoma analyzed for VWF (green), TIE2 (red), and CD11b (blue). Arrowheads indicate TIE2+CD11b+VWF− monocytes. (E) Pancreatic adenocarcinoma analyzed for CD16 (green), TIE2 (red), and CD14 (blue). High-magnification photos show the presence of TIE2+CD16+CD14+ monocytes (arrows) in the tumor stroma. (F) Colon adenocarcinoma analyzed for CD14 (green), TIE2 (red), and CD11b (blue). A triple-positive CD14+TIE2+CD11b+ TEM with peri-endothelial localization is indicated by the arrow. Note the presence of CD14−TIE2−CD11b+ inflammatory cells (arrowheads). Scale bars as indicated. (G) TIE2 expression in nonneoplastic tissues is restricted to vascular ECs. Nonneoplastic colon mucosa adjacent to tumor tissue analyzed by confocal immunofluorescence staining of CD31 or CD14 (green), TIE2 (red), and CD45 (blue). Note that the lamina propria macrophages are CD14−TIE2−. A single CD14+TIE2− monocyte (arrow) is found within a TIE2+ blood vessel. Tonsil sections show that TIE2 expression (red) is restricted to CD31+ vascular ECs (green). CD19+ B cells are stained in blue. Scale bars as indicated.
Confocal immunofluorescence analysis of human cancer sections confirms the presence of TIE2+CD45+CD14+ tumor-infiltrating monocytes. (A) Colon adenocarcinoma analyzed for CD31 (green), TIE2 (red), and CD45 (blue). Confocal planes are shown individually and after merging. Several TIE2+CD45+CD31− hematopoietic cells (merge of red and blue giving purple; arrows) are found within the tumor stroma. Note that TIE2 is expressed by vascular ECs (merge of green and red giving yellow; arrowheads), which are TIE2+CD45−CD31+. (B) Gastric adenocarcinoma analyzed for CD31 (green), TIE2 (red), and CD45 (blue). Some TIE2+CD45+CD31− hematopoietic cells are found in the tumor stroma (arrows) together with TIE2+CD45−CD31+ tumor blood vessels (arrowhead). Scale bars as indicated. (C) Colon adenocarcinoma analyzed for CD14 (green), TIE2 (red), and CD11b (blue). Several TIE2+CD14+CD11b+ monocytes (arrows) are found within the tumor stroma. Note TIE2 expression by TIE2+CD14−CD11b− vascular ECs (arrowheads). (D) Gastric adenocarcinoma analyzed for VWF (green), TIE2 (red), and CD11b (blue). Arrowheads indicate TIE2+CD11b+VWF− monocytes. (E) Pancreatic adenocarcinoma analyzed for CD16 (green), TIE2 (red), and CD14 (blue). High-magnification photos show the presence of TIE2+CD16+CD14+ monocytes (arrows) in the tumor stroma. (F) Colon adenocarcinoma analyzed for CD14 (green), TIE2 (red), and CD11b (blue). A triple-positive CD14+TIE2+CD11b+ TEM with peri-endothelial localization is indicated by the arrow. Note the presence of CD14−TIE2−CD11b+ inflammatory cells (arrowheads). Scale bars as indicated. (G) TIE2 expression in nonneoplastic tissues is restricted to vascular ECs. Nonneoplastic colon mucosa adjacent to tumor tissue analyzed by confocal immunofluorescence staining of CD31 or CD14 (green), TIE2 (red), and CD45 (blue). Note that the lamina propria macrophages are CD14−TIE2−. A single CD14+TIE2− monocyte (arrow) is found within a TIE2+ blood vessel. Tonsil sections show that TIE2 expression (red) is restricted to CD31+ vascular ECs (green). CD19+ B cells are stained in blue. Scale bars as indicated.
In agreement with flow analysis, we found that the nonneoplastic tissues adjacent to the tumors contained only a few TIE2+ hematopoietic cells (Figure 6G). Moreover, in normal tissues obtained from surgery, we found that TIE2 expression was restricted to ECs, even in organs heavily infiltrated by hematopoietic lineage cells, such as the tonsils (Figure 6G). Taken together, these findings indicated that human tumors selectively recruit a population of TIE2-expressing CD14+ monocytes distinct from common macrophages (TAMs) and reminiscent of mouse TEMs.15
Angiopoietin-2 exerts chemotactic activity on TIE2-expressing monocytes
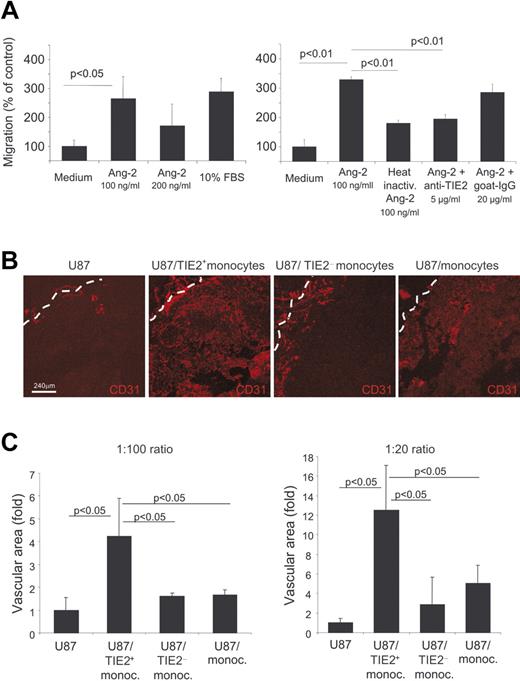
Ang-1 and Ang-2 stimulate vascular morphogenesis and shape adult angiogenesis by promoting EC chemotaxis, survival, and/or apoptosis in a context-dependent manner and in cooperation with other angiogenic factors. We then investigated whether Tie2 expressed by monocytes could impart similar biologic responses also to these cells. Previous studies showed that Ang-2 stimulated the migration of blood-derived endothelial-like cells more efficiently than Ang-1.36 Using a modified Boyden chamber assay, we analyzed cell migration in response to an Ang-2 gradient. We isolated resident monocytes, which are enriched in TEMs, and inflammatory monocytes by magnetic sorting (see Cell purification and cell sorting in Materials and methods and Figure S1). As shown in Figure 7A, both serum and Ang-2 induced significant migration of resident monocytes (P < .05 vs control medium), with the higher response to an Ang-2 concentration of 100 ng/mL. Conversely, Ang-2 showed no significant chemotactic activity on inflammatory monocytes (data not shown). To verify that the chemotactic response observed was promoted by the specific interaction between Ang-2 and TIE2, the cells were pretreated with neutralizing anti-TIE2 antibodies. Anti-TIE2 antibodies, but not control immunoglobulins, significantly blocked Ang-2–induced cell migration. Of note, heat inactivation of Ang-2 abolished its chemotactic activity. Together, these results strongly suggest that TIE2 expressed on monocytes mediated their migration in response to Ang-2.
Circulating TIE2+ monocytes migrate toward Ang-2 and have marked proangiogenic activity in vivo. (A) Modified Boyden chamber assays show migration of resident monocytes toward Ang-2. The 2 graphics show parts of 2 independent experiments of 3 performed. Both serum and Ang-2 induced significant migration of resident monocytes (left histograms; P < .05 vs control: Medium). Heat inactivation of Ang-2 or treatment of the cells with neutralizing anti-TIE2 antibodies, but not with control immunoglobulins, abrogates cell migration in response to Ang-2 (right histograms). (B) Human glioma U87 cells were injected subcutaneously into nude mice with or without the indicated monocyte populations at a 1:100 or 1:20 ratio. Tumors were grown for 5 to 7 days and analyzed by CD31 immunostaining and confocal analysis to assess angiogenesis. Representative pictures of the tumors are shown. The tumor margin is indicated by a dashed line. (C) The mean vascular area (n = 3 tumors/group) was calculated by digital image analysis and expressed as fold increase over the value obtained in tumors from U87 cells only. Error bars indicate SD. Statistical difference between groups was calculated by Student t test.
Circulating TIE2+ monocytes migrate toward Ang-2 and have marked proangiogenic activity in vivo. (A) Modified Boyden chamber assays show migration of resident monocytes toward Ang-2. The 2 graphics show parts of 2 independent experiments of 3 performed. Both serum and Ang-2 induced significant migration of resident monocytes (left histograms; P < .05 vs control: Medium). Heat inactivation of Ang-2 or treatment of the cells with neutralizing anti-TIE2 antibodies, but not with control immunoglobulins, abrogates cell migration in response to Ang-2 (right histograms). (B) Human glioma U87 cells were injected subcutaneously into nude mice with or without the indicated monocyte populations at a 1:100 or 1:20 ratio. Tumors were grown for 5 to 7 days and analyzed by CD31 immunostaining and confocal analysis to assess angiogenesis. Representative pictures of the tumors are shown. The tumor margin is indicated by a dashed line. (C) The mean vascular area (n = 3 tumors/group) was calculated by digital image analysis and expressed as fold increase over the value obtained in tumors from U87 cells only. Error bars indicate SD. Statistical difference between groups was calculated by Student t test.
TIE2-expressing monocytes are proangiogenic
We previously showed that mouse TEMs promote angiogenesis.15 To investigate whether human TIE2+ monocytes have proangiogenic activity, we isolated CD14+TIE2+ and CD14+TIE2− cells from human PB by cell sorting and coinjected these cells in increasing ratios (1:100 and 1:20) with U87 human glioma cells subcutaneously in nude mice. As controls, we injected U87 cells alone and U87 cells together with unfractionated CD14+ monocytes, which mostly comprise inflammatory monocytes. We studied tumor vascularization 5 or 7 days after injection, when tumors were at an early stage of growth (Figure 7B).
In tumors derived from the injection of U87 cells alone (n = 6), CD31+ blood vessels were exceedingly scarce within the inner tumor mass, whereas few large blood vessels, likely sequestered from the subcutaneous space, surrounded the tumors (Figure S2). This finding indicates that angiogenesis had not yet started at this early time of tumor growth. On the contrary, tumors coinjected with human CD14+TIE2+ monocytes (n = 6) were larger and much more vascularized, with a profuse vascular framework appreciably extending from the tumor periphery toward the inner mass. In these tumors, blood vessels had the typical morphology of angiogenic vessels (Figure 7B). In tumors coinjected with unfractioned CD14+ (n = 6) or CD14+TIE2− monocytes (n = 6), a small rim of blood vessel ingrowths lined the tumor periphery, but only a few spots of angiogenic vessels were observed within the inner mass. Computer-assisted digital image analysis27 showed that the overall vascular area was significantly greater in tumors coinjected with CD14+TIE2+ monocytes than in control tumors and tumors coinjected with unfractioned or TIE2+ monocyte-depleted CD14+ cells at both cell ratios (Figure 7C). These results indicated that, among human blood monocytes, the CD14+TIE2+ subset was specifically endowed with the ability to enhance angiogenesis in a tumor transplantation model.
Discussion
It has been proposed that tumor-infiltrating innate immune cells are more likely to contribute to tumor progression than they are to mount an effective host antitumor response.1,2,5 Among inflammatory cells found in tumors, TAMs and mast cells are thought to support tumor growth and neovascularization by producing a wide array of growth and proangiogenic factors. Many of these molecules can be up-regulated in macrophages by tumor-secreted factors and by hypoxia, which is a hallmark of cancer.10 More recently, hematopoietic cells phenotypically distinct from TAMs have been implicated in tumor angiogenesis.7,15,17,20–26 In some studies, the impaired recruitment of such hematopoietic populations was sufficient to restrict tumor angiogenesis and growth, suggesting that the predominant proangiogenic activity of hematopoietic cells in experimental tumors might be attributed to yet poorly characterized myeloid subpopulations rather than TAMs.15,17,20,21,24
In our previous studies, we showed that proangiogenic Tie2-expressing monocytes are recruited to mouse tumors, where they constitute a small fraction of the total CD11b+ tumor-infiltrating myeloid cells. Because TEMs isolated from PB were proangiogenic, we proposed the notion that TEMs may have inherent vascular growth–promoting activity, thus representing a circulating reservoir of cells committed to a proangiogenic function.15 In this work, we describe a novel subset of proangiogenic monocytes found in human PB and tumors. These cells express a unique combination of cell-surface markers (TIE2+CD14lowCD16+CCR2−L-selectin−), which distinguishes them from common inflammatory monocytes.14 The phenotype and proangiogenic activity of human TEMs (TEMs) are reminiscent of those of previously described murine TEMs.15 However, the substantial lack of surface markers shared by human and mouse monocytes, together with the paucity and elusive phenotype of monocytes in mice, make it difficult to ascertain whether the TIE2+ cells that we identified in PB (this study and De Palma et al15 ) represent the same cell type in humans and mice. Moreover, the lack of monocyte- and macrophage-specific markers in mice prevented us from accurately defining the phenotype of TEMs, which could be distinguished from common TAMs only based on Tie2 expression.
Here, we show that TEMs are distinct from TAMs according to their surface-marker profile, which conversely overlaps with that of resident monocytes, a population of immature monocytes believed to represent precursors of tissue macrophages.14 Remarkably, we found that TEMs were recruited to human carcinomas and soft-tissue sarcomas and specifically promoted angiogenesis in experimental tumors in vivo, indicating a novel and unexpected role for resident monocytes in tumor angiogenesis. Of note, only a fraction of the circulating resident monocytes expressed TIE2, suggesting that a certain degree of phenotypical and functional heterogeneity exists within the resident monocyte subset. Interestingly, our studies showed that inflammatory monocytes, which represent the major monocyte population in PB and are believed to represent the precursors of TAMs, were less proangiogenic than TEMs in vivo.
A peculiar feature of TEMs is that they preferentially localize in the proximity of tumor blood vessels, which is consistent with their proangiogenic activity in transplantation assays (this study and De Palma et al15 ). However, the molecular bases of the proangiogenic activity of TEMs and the factors that recruit these cells to tumors remain to be investigated. Circulating TEMs did not express L-selectin (CD62L), a member of the selectin family of adhesion molecules that facilitate rolling of inflammatory leukocytes along the vascular endothelium, or CCR2, the receptor for MCP-1 (monocyte chemoattractant protein-1, also known as CCL2), a chemokine involved in the recruitment of inflammatory monocytes to tumors and experimentally induced inflammation. Although we did not investigate recruitment of TEMs to inflammatory disease, their L-selectin−CCR2− phenotype should exclude them from inflamed tissues. In this regard, recruitment of proangiogenic myeloid cells to tissues harboring an inducible VEGF transgene has been shown to occur in the absence of inflammatory stimuli.25 Thus, noninflammatory circuits may govern the recruitment of TEMs to tumors. It is tempting to speculate that TEMs may use selected tumor-secreted, noninflammatory mediators to specifically home to tumors, even at early stages of tumor progression, a feature that would distinguish these cells form other tumor-infiltrating hematopoietic populations, such as TAMs, which are prototypical cellular components of the inflammatory network in established tumors. According to this hypothesis, TEMs may be attracted to yet avascular and noninflamed tumor areas in a CCR2-independent manner, where they would promote the angiogenic process. The tumor-homing specificity of TEMs could be mediated by signals that are produced by the cancer cells or stromal components of tumors, including activated myofibroblasts37 and ECs.38 Peri-tumoral blood vessels may indeed express selected adhesion molecule and chemoattractant factors for monocytes.39–41 Among these factors, angiopoietins may have a crucial role. We demonstrated that TEMs directly respond to Ang-2 and that the TIE2 receptor is involved in this response. Expression of Ang-2, which is stored in Weibel-Palade bodies in ECs and rapidly released from them upon EC activation,42,43 is indeed up-regulated by tumor hypoxia and may function as a chemoattractant for TIE2+ monocytes. Intriguingly, we showed that TIE2 expression was up-regulated in tumor-infiltrating TEMs, which is consistent with the reported up-regulation of this receptor in ECs at angiogenic and hypoxic sites44,45 and suggests the occurrence of a positive feedback loop reinforcing TEM recruitment at these sites.
We did not observe TEMs in normal, nonneoplastic tissues, suggesting that they may represent a specific subset of resident monocytes distinct from the precursors of tissue macrophages. It is conceivable, although experimental evidence in this direction is still lacking, that resident monocytes can be further divided into subsets endowed with tissue or organ specificity,14 including tropism for neoangiogenic sites, such as growing and regenerating tissues, wounds, and tumors. Because proangiogenic TEMs circulate in PB at steady state, they may have a constitutive role in supporting tissue growth and regeneration, as we previously noted in hepatectomized mice.17 It is also possible, however, that a slow turnover of tissue resident macrophages prevented us from detecting a role of TEMs as precursors of macrophages in the organs analyzed, and further studies are thereafter required.
Although TIE2 should also be expressed by rare CECs and EPCs, TEMs can be easily distinguished from CECs/EPCs because the latter cells do not express hematopoietic markers. However, in agreement with a recent study,31 we noted that a very small percentage of TIE2+ monocytes also expressed VEGFR-2, an endothelial-specific receptor commonly used to identify CECs/EPCs. Although our analyses showed that greater than 95% of the CD14+TIE2+ cells were VEGFR-2−, we cannot formally rule out that a certain degree of heterogeneity may exist in the “TEM” population and that minor cell subsets copurifying with TEMs might contribute to some of the biologic activities observed. Kerbel and colleagues (Mancuso et al29 and Shaked et al46 ) have proposed that the frequency of CECs/EPCs in PB may serve as a pharmacodynamic surrogate marker to monitor the magnitude of angiogenesis and the effectiveness of antiangiogenic therapy. It will be worth investigating whether TEMs are reproducibly increased in cancer patients according to the type and stage of the disease and in other pathologic conditions characterized by dysregulated angiogenesis. In such a circumstance, TEMs could provide a novel, robust, and biologically relevant marker to monitor angiogenesis.
The identification of TEMs opens a number of avenues to the development of both antiangiogenic and proangiogenic therapy. TEMs may represent unrecognized targets of cytoablative treatments, which may restrict tumor growth not only by targeting cancer cells but also by suppressing myelopoiesis and, consequently, impairing the generation and function of proangiogenic monocytes.47 The identification of TEM-specific genes will provide candidate molecular targets for the development of new and safer antiangiogenic therapy. On a different perspective, TEMs could be administered or recruited to ischemic sites to promote angiogenesis and improve organ function.
An Inside Blood analysis of this article appears at the front of this article.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to C. Staudacher for provision of surgical specimens; E. Hauben for initial help with cell purification; A. Palini and V. Vecchio (cytometry facility) for cell sorting; and P. Ghia for access to flow cytometry equipment (Coulter). M.D.P. is a recipient of an Associazione Italiana per la Ricerca sul Cancro (AIRC) fellowship.
This study was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC 52-2005), EU (Tumour-host genomics, LSHC-CT-2005-518198), and Telethon (TIGET; L.N.).
Authorship
Contribution: M.A.V. designed research with M.D.P. and L.N., performed research, and analyzed data. M.P. and C.D. performed immunohistochemistry on cancer specimens. F.P. performed real-time PCR studies. C.S. performed Western-blot analysis. E.Z. and R.M. performed cell migration assays. M.D.P. designed and supervised research, analyzed data, and wrote the manuscript. L.N. designed and supervised research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
M.A.V. and M.D.P. contributed equally to this work.
Correspondence: Luigi Naldini and Michele De Palma, San Raffaele Telethon Institute for Gene Therapy, via Olgettina, 58, 20132 Milano, Italy; e-mail: naldini.luigi@hsr.it and depalma.michele@hsr.it.