Abstract
CD99 is a long-known leukocyte antigen that does not belong to any of the known protein families. It was recently found on endothelial cells, where it mediates transendothelial migration of human monocytes and lymphocyte recruitment into inflamed skin in the mouse. Here, we show that CD99L2, a recently cloned, widely expressed antigen of unknown function with moderate sequence homology to CD99, is expressed on mouse leukocytes and endothelial cells. Using antibodies, we found that CD99L2 and CD99 are involved in transendothelial migration of neutrophils in vitro and in the recruitment of neutrophils into inflamed peritoneum. Intravital and electron microscopy of cremaster venules revealed that blocking CD99L2 inhibited leukocyte transmigration through the vessel wall (diapedesis) at the level of the perivascular basement membrane. We were surprised to find that, in contrast to CD99, CD99L2 was not relevant for the extravasation of lymphocytes into inflamed tissue. Although each protein promoted cell aggregation of transfected cells, endothelial CD99 and CD99L2 participated in neutrophil extravasation independent of these proteins on neutrophils. Our results establish CD99L2 as a new endothelial surface protein involved in neutrophil extravasation. In addition, this is the first evidence for a role of CD99 and CD99L2 in the process of leukocyte diapedesis in vivo.
Introduction
Leukocytes are recruited into inflamed tissue via endothelial adhesion molecules and chemoattractants.1–4 Upon docking to the endothelial surface, which is mediated mainly via selectins and leukocyte integrins, leukocytes transmigrate through the vessel wall, a process called diapedesis.5,6 Leukocytes have been reported to traverse the endothelial cell layer on either a transcellular or a paracellular junctional route.7,8 Almost all known endothelial membrane and adhesion molecules that participate in the diapedesis process are located at endothelial cell contacts and are therefore likely to participate in the junctional migration of leukocytes.
Vascular/endothelial cadherin (VE-cadherin) represents a barrier on this route, because adhesion-blocking antibodies against VE-cadherin accelerate recruitment of leukocytes into inflamed tissue.9 In contrast to VE-cadherin, all other endothelial cell contact proteins assist in the diapedesis process. All of these proteins except one are members of the immunoglobulin supergene family (Ig-SF), such as platelet-endothelial cell adhesion molecule (PECAM)-1,10,11 members of the junctional adhesion molecule (JAM) family,12–14 endothelial cell-selective adhesion molecule (ESAM),15 intercellular adhesion molecule (ICAM)-216 and polio virus receptor (PVR),17 a member of the nectin family. It is not yet known how they function in detail and it will be a challenging goal for the future to elucidate potential cascades and the interplay of these endothelial cell contact proteins during leukocyte diapedesis.
One of the most recently identified proteins participating in leukocyte diapedesis is CD99, a long-known leukocyte surface antigen that was found to be expressed at endothelial cell contacts and to participate in the in vitro transmigration of human monocytes through monolayers of human endothelial cells.18 That CD99 is indeed relevant for leukocyte extravasation in vivo was demonstrated in the mouse. Antibodies against mouse CD99 inhibited the recruitment of lymphocytes into inflamed sites of the skin.19 Whether CD99 would be relevant for the extravasation of myeloid cells in vivo has not yet been analyzed.
CD99 is a rather small membrane protein with a highly O-glycosylated extracellular part comprising only approximately 100 amino acids. Its primary structure indicates that it is not related to any known protein family. Three other genes have been described, 2 of which are mapping in the vicinity of the CD99 gene in the human genome and displaying moderate sequence homology to CD99. One of them (MIC2R) is a pseudogene; the second, PBDX, codes for an erythrocyte cell surface antigen (Xga) of unknown function. The third related gene coding for a protein of unknown function was recently cloned in various species.20 Sequencing revealed a homology of not more than 32% amino acid identity between this gene and CD99 in the mouse. Based on in situ hybridizations, the mRNA seems to be widely distributed, with prominent expression on neuronal cells, choroid plexus, Sertoli cells, and granulosa and theca cells of the ovary.20 Although no studies have been performed that would attribute any function to the corresponding protein and expression on leukocytes has not been analyzed, it was tentatively named CD99 antigen like-2 (CD99L2).
Here, we have analyzed whether CD99L2 would indeed be functionally related to CD99 and whether it would be relevant for the process of leukocyte extravasation. We generated antibodies against mouse CD99L2 with which we found that this antigen is expressed on neutrophils and lymphocytes as well as on endothelium in various tissues. Despite its strong expression on lymphocytes and in contrast to CD99, CD99L2 was not involved in the recruitment of lymphocytes into inflamed skin. However, in 2 different inflammation models, antibodies against CD99L2 inhibited the extravasation of neutrophils. Intravital and electron microscopy allowed us to demonstrate that CD99L2 was not relevant for the contact formation between leukocytes and endothelium but rather for the actual transmigration step. In addition, CD99 was also found to participate in neutrophil diapedesis in vivo. Our results establish CD99L2 as a new endothelial surface protein that participates in the extravasation and diapedesis of neutrophils.
Materials and methods
Cell culture
The following cells were propagated as described: protein lipid protein-specific TH1 memory/effector T cell line SJL.PLP7,21 Chinese hamster ovary (CHO) cells,22 bEnd.5 mouse endothelioma cells,23 and human umbilical vein endothelial cells.24 CHO cell lines expressing the E3-E4 form or the E3A-E3-E4 form of mouse CD99L2 or the respective CD99L2-Fc fusion protein were generated by electroporation according to established procedures.25 COS-7 cells were transiently transfected with mouse CD99 full length or the E3-E4 form of CD99L2 full length according to the manufacturer's protocol (GeneJammer; Stratagene, La Jolla, CA).
Antibodies
Polyclonal rabbit antisera against mouse CD99L2 (E3-E4 form) were generated against the CD99L2s-Fc fusion protein. Antibodies against the IgG1-Fc part were removed from the serum by incubation with human IgG1-coupled to CNBr-activated Sepharose (GE Healthcare, Freiburg, Germany). Specific antibodies against CD99L2 were affinity-purified with CD99L2-Fc immobilized on CNBr-Sepharose. F(ab′)2 fragments were generated with immobilized pepsin on beads (Pierce, Rockford, IL) according to the manufacturer's protocol. Uncleaved IgG and Fc fragments were removed using protein A Sepharose (GE Healthcare). Purity (< 0.3% intact IgG) and proper size of the F(ab′)2 fragments were confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Relative binding efficiency was tested by enzyme-linked immunosorbent assay using CD99L2-Fc as antigen. No endotoxin was detectable. Affinity-purified polyclonal rabbit antibodies against mouse CD99 and mouse ESAM were generated as described previously.25 Monoclonal antibodies were purified from hybridoma supernatants: anti-mouse ICAM-1 (YN1/1.7, rat IgG-2b; American Type Culture Collection, Manassas, VA)26 ; anti mouse L-selectin (MEL14; American Type Culture Collection); anti-mouse P-selectin (RB40.34, rat IgG1); anti-mouse PECAM-1 (1G5.1, rat IgG2b; 5D2.6, rat IgG2a) (both unpublished). Purchased antibodies: anti-LFA-1 (BD Pharmingen, San Diego, CA). Cy3-conjugated anti-rabbit IgG, PE-conjugated anti-rabbit IgG, peroxidase-conjugated anti-rabbit IgG (Dianova, Hamburg, Germany); fluorescein isothiocyanate-labeled and peroxidase-labeled polyclonal antibodies to rabbit IgG F(ab′)2 (Acris, Hiddenhausen, Germany). Immunofluorescence staining and Western blots were done as described previously.25
Glycosidase treatment
Three micrograms of CD99L2-Fc fusion protein as digested in 150 μl of 25 mM NaH2PO4, pH 6.0, for 18 hours at 37°C. The reaction was stopped by boiling in SDS-PAGE sample buffer; 100 ng of the reaction mixture were electrophoresed, and detected by immunoblotting with peroxidase conjugated anti-human IgG followed by ECL.
Cloning of mouse CD99L2 isoforms
For generation of a mouse CD99L2-Fc fusion protein, total cellular RNA was isolated from mouse spleen cells using the TRIzol reagent (Invitrogen) according to the manufacturer's protocol. A cDNA fragment coding for the extracellular part (amino acid 1-161) of the E3—E4 form of mouse CD99L2 (GenBank accessopm number NM_138309) was generated by reverse transcription-polymerase chain reaction (RT-PCR) from total spleen RNA, using a HindIII site-containing sense oligonucleotide (5′-TAGTAGAAGCTTCTGCGCCTGCTCCCAGCCAT-3′) and an EcoRI site-containing antisense oligonucleotide (5′-CTACTAGAATTCACTTACCTCCAGTTTCTGTCGATATGCC-3′). The product was inserted into a pcDNA3-based Fc-construct vector (pcDNA3/hlgG-Fc) in frame and upstream of a fragment of human IgG1 covering bases 553 to 1803 (hinge, CH2, CH3). A full-length cDNA for mouse CD99L2 was generated by RT-PCR from total RNA from mouse spleen using the sense oligonucleotide 5′-CTCCTGCGCTAACTGTGCTCCT-3′ and the antisense oligonucleotide 5′-AATCCGGGGTGGCTCGGGTGGT-3′. The PCR product was inserted into pcDNA3 (Invitrogen).
For detection of the splice variants of CD99L2 in endothelial cells, the following primers were used: For mouse CD99L2: sense primer, 5′-CTCCTGCGCTAACTGTGCTCCT; antisense primer, 5′-GGTCACAAGCGGTGGCTTCCT; for human CD99L2: sense primer, 5′-GCTCTCGCGCTAACTGTGCTC; antisense primer, 5′-GTGGCACCATTGTGCATGCCTG. RT-PCR was performed with total cell RNA from mouse endothelioma cells bEnd.5 and from HUVEC.
Immunohistochemistry and flow cytometry
Animals were anesthetized using isoflurane anesthesia (Abbott, Wiesbaden, Germany), and were perfused with 10 mL of phosphate-buffered saline (PBS) through the left ventricle of the heart. Tissue was removed, embedded in Tissue-Tek OCT Compound (Miles, Giessen, Germany), and snap-frozen. Cryostat sections (6 μm) were stained as described.27 Flow cytometry was done as described.28
Aggregation assay
Our assay was modified from a method described previously29 and was performed as described previously.19 Quantification of aggregation was estimated by the following formula: % aggregation = (N0 − Nt)/N0 × 100, where Nt is the total number of particles at the incubation time t, and N0 is the total number of cells. In some experiments, a mixed population of untransfected and CD99L2-transfected CHO cells was subjected to cell aggregation assays. One cell type had been labeled (20 μmol/L /5 × 106) with Cell Tracker Green 5-chloromethylfluorescein diacetate (Invitrogen), and the number of cells per each cell type in aggregate was counted in a fluorescence microscope.
Adhesion assay
Adhesion assays were done as described previously,19 except that PMNs were used instead of lymphocytes.
Preparation of mouse bone marrow neutrophils
NMRI mice (Harlan-Winkelmann, Borchen, Germany) were killed, femurs and tibias were cut out, and muscles were removed. Bones were placed in Ca2+/Mg2+-free HBSS (HBSS−/−; Invitrogen) to prevent dry-out. The ends of the bones were cut, and the bone marrow was flushed into a 50-ml tube with HBSS−/− using a short (5/8-inch) 25 G needle and a 2-mL syringe. Larger bone marrow pieces were disaggregated using a plastic pipette and a mesh (70 μm). The cell suspension was centrifuged (at 300g, 10 minutes) and the pellet was resuspended in 1 mL of HBSS−/−. The cell suspension was then added on top of a 10-mL tube containing 4 mL of Histopaque 1119 and 4 mL of Histopaque 1077 (Sigma-Aldrich, Steinheim, Germany). After centrifugation for 30 minutes at 700g (brake off), the polymorphonuclear cell (PMN)-containing cell layer was collected and washed 2 times with HBSS−/−.
Transmigration assay
Transendothelial migration of SJL.PLP7 and PMNs was performed as described previously.19,23 bEnd.5 cells (5 × 104cells/well in 6.5 mm Transwells; Costar/Corning, Bodenheim, Germany) were grown for 2 days for each assay. For assays with PMNs, 5 × 105 PMNs were added per transwell, and chemoattraction was done with 40 ng/mL keratinocyte-derived chemokine (KC) in the bottom chamber. Migrated leukocytes were collected for cell counting (CASY; Schärfe-System, Reutlingen, Germany). Confluence of the endothelial monolayer was confirmed after each assay on formalin-fixed, 2.6% Giemsa-stained inserts.
Contact hypersensitivity and T-cell immigration into inflamed skin
Experiments were performed as described previously.19
Thioglycollate-induced peritonitis assay
For each assay, 5 female BALB/c mice, 8-12 weeks old, were used. Thioglycollate-induced peritonitis assays (induced by 3% sterile thioglycollate broth) were essentially performed as described previously,28 except that the peritoneal lavage was done with 20 mL of PBS containing 3 mM EDTA; total cell counts were determined with a cell counter, and the percentage of neutrophils was determined by flow cytometry (FACSCalibur; BD Biosciences, Heidelberg, Germany) using the monoclonal antibody RB6-8C5 against Ly-6G and Ly6C (Gr-1).
Intravital microscopy
Surgical preparation of cremaster muscles and intravital microscopy were essentially done as described previously.15,30 Inflammatory stimulation was achieved by intrascrotal injection of 50 ng rmIL-1β (R&D Systems, Minneapolis, MN) diluted in 0.3 mL PBS, administered to mice 4 hours before microscopic observation (n = 6 each group). At the same time, 50 μg affinity-purified rabbit IgG (against CD99 or CD99L2) or control rabbit IgG or 75 μg F(ab′)2-fragments were injected intravenously. For each animal, 3-5 single unbranched postcapillary venules with diameters of 17 to 35 μm were analyzed.
Electron microscopy
Four hours after inflammatory stimulation and antibody injection (as described previously), the cremaster muscle was dissected and prepared for electron microscopy as described previously for other tissues.31 For each vessel, the number of leukocytes in each of the following positions was determined: A, within lumen of vessel; B, crossing the endothelium; C, between endothelium and basement membrane/pericyte; D, outside the vessel but within 50 μm of it. The fraction of leukocytes that were diapedesing but still inside the basement membrane or pericyte layer was calculated by the equation C/C+D. For each antibody type, 32 to 52 vessels of 2 to 3 mice were evaluated.
Results
Generation and characterization of specific antibodies against CD99L2
In the course of cloning the mouse ortholog of human CD99,19 we found additional clones in the mouse gene data base that coded for a CD99-related protein (MIC2-like1), for which the name CD99L2 was later suggested.20 Based on the sequence of clone NM_138 309, we designed primers with which we cloned 2 RT-PCR products of 660 base pairs (bp) and 729 bp from poly A+-RNA of mouse spleen. Sequencing the smaller product revealed full match with clone NM_138 309. The larger product was identical except for an additional 69-bp insertion. During the course of our project, this larger form has been described in the gene data base, and the inserted sequence has been designated as exon 3A.20
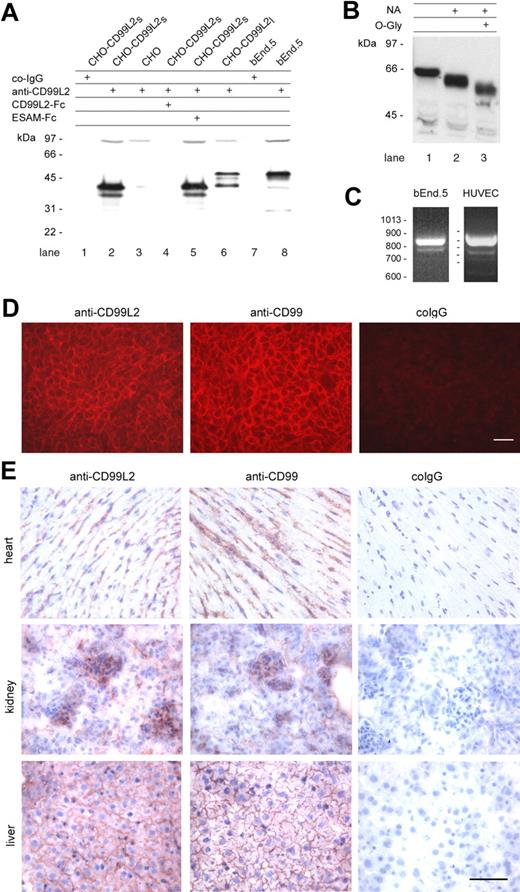
To study the CD99L2 protein, we generated polyclonal antibodies against a mouse CD99L2-Fc fusion protein containing the complete extracellular part of the shorter splice variant of CD99L2 (E3-E4 form) fused to the Fc part of human IgG1. Polyclonal antibodies against mouse CD99 have been raised against a CD99-Fc fusion protein and have been described previously.19 Because mouse CD99L2 and CD99 show 32% amino acid identity, we tested our affinity-purified antibodies against CD99L2 and CD99 for cross-reactivity. The antibody preparations had been extensively depleted for antibodies directed against the Fc-part of the fusion proteins. As shown in Figure 1A, anti-CD99L2 antibodies detected only CD99L2-Fc but not CD99-Fc or ESAM-Fc in immunoblots. Likewise, anti-CD99 antibodies recognized CD99-Fc, but not CD99L2-Fc or ESAM-Fc (Figure 1A). Furthermore, COS-7 cells transfected with CD99L2 were brightly stained with anti-CD99L2 antibodies, whereas only background staining was observed with CD99-transfected cells. Likewise, anti-CD99 antibodies stained COS-7 cells transfected with CD99, but no signal was obtained when cells were transfected with CD99L2 (Figure 1B). Thus, our affinity-purified antibodies against CD99L2 and CD99 are strictly specific for their respective antigen.
Anti-CD99L2 and anti-CD99 antibodies specifically react only with their corresponding antigen. (A) Immunoblots of CD99-Fc, CD99L2S-Fc, and ESAM-Fc (as indicated above) with affinity-purified antibodies against CD99 and CD99L2 (as indicated below). (B) Immunofluorescence staining of COS-7 cells transiently transfected with full-length CD99L2s (top panel) or full-length CD99 (bottom panel) using affinity-purified antibodies against CD99 or CD99L2 (as indicated on the left). Bar = 25 μm.
Anti-CD99L2 and anti-CD99 antibodies specifically react only with their corresponding antigen. (A) Immunoblots of CD99-Fc, CD99L2S-Fc, and ESAM-Fc (as indicated above) with affinity-purified antibodies against CD99 and CD99L2 (as indicated below). (B) Immunofluorescence staining of COS-7 cells transiently transfected with full-length CD99L2s (top panel) or full-length CD99 (bottom panel) using affinity-purified antibodies against CD99 or CD99L2 (as indicated on the left). Bar = 25 μm.
CD99L2 is expressed on endothelial cells
To characterize the proteins corresponding to the 2 splice variants of CD99L2, CHO cells transfected with the short (CD99L2S) and the long (CD99L2L) splicing variants of CD99L2 were analyzed by immunoblotting. This revealed that CD99L2S gave rise to a 40-kd/37-kd doublet, whereas the longer form was recognized as a 56-kd/40-kd doublet (Figure 2A). Specificity of the signals was confirmed by blocking studies with the CD99L2-Fc fusion protein. It is likely that the smaller molecular weight bands detected in each case in transfected CHO cells represent either degradation products or glycosylation variants, due to ectopic expression. Because the sequence-based calculation of the molecular weight of the 2 splicing variants would predict sizes of 23.5 and 26.1 kd, respectively, CD99L2 was likely to be glycosylated. The lack of N-glycosylation sites suggests O-glycosylation. Indeed, treatment of CD99L2 with neuraminidase followed by O-glycosidase caused a shift in molecular weight, demonstrating O-glycosylation (Figure 2B).
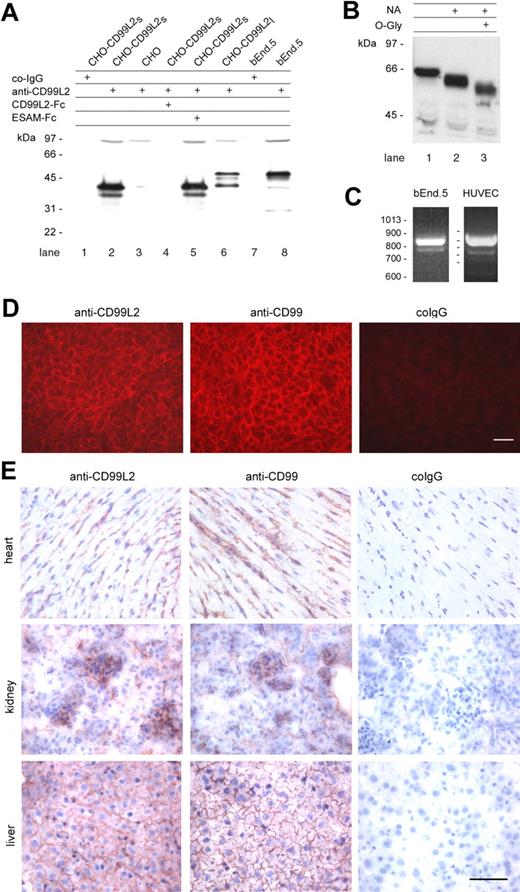
Expression of CD99L2 on endothelial cells. (A) Immunoblots of cell lysates of CHO cells stably transfected with the short E3—E4 splice variant (CD99L2s; lanes 1-2 and 4-5), with the long E3A-E3-E4 splice variant of mouse CD99L2 (CD99L2l; lane 6), or mock-transfected (lane 3), and of lysates of bEnd.5 endothelioma cells (lanes 7-8) were analyzed with preimmune serum IgG (co-IgG, lanes 1 and 7) or affinity purified antibodies against CD99L2 (anti-CD99L2, lanes 2-6, 8). To control the specificity anti-CD99L2 antibodies were preincubated either with recombinant CD99L2-Fc (lane 4) or with ESAM-Fc (lane 5). (B) Purified CD99L2-Fc was digested without enzymes or with neuraminidase (NA) or with neuraminidase and O-glycosidase (O-Gly), followed by immunoblotting with peroxidase-conjugated anti human IgG antibodies. (C) Detection of CD99L2 splice variants in bEnd.5 endothelioma cells and HUVEC by RT-PCR. (D) Immunofluorescence staining of bEnd.5 endothelioma cells with affinity purified antibodies against CD99L2 and negative control IgG from the respective preimmune serum (as indicated). First antibodies were detected with Cy3-conjugated secondary antibodies and visualized by fluorescence microscopy. Bar = 20 μm. (E) Immunoperoxidase staining of cryostat sections of mouse heart, kidney, and liver with affinity-purified antibodies against mouse CD99L2, mouse CD99, and control rabbit IgG from preimmune serum (as indicated). Tissue sections were incubated with first antibodies, followed by washing and incubation with secondary and tertiary reagent. Bar = 70 μm
Expression of CD99L2 on endothelial cells. (A) Immunoblots of cell lysates of CHO cells stably transfected with the short E3—E4 splice variant (CD99L2s; lanes 1-2 and 4-5), with the long E3A-E3-E4 splice variant of mouse CD99L2 (CD99L2l; lane 6), or mock-transfected (lane 3), and of lysates of bEnd.5 endothelioma cells (lanes 7-8) were analyzed with preimmune serum IgG (co-IgG, lanes 1 and 7) or affinity purified antibodies against CD99L2 (anti-CD99L2, lanes 2-6, 8). To control the specificity anti-CD99L2 antibodies were preincubated either with recombinant CD99L2-Fc (lane 4) or with ESAM-Fc (lane 5). (B) Purified CD99L2-Fc was digested without enzymes or with neuraminidase (NA) or with neuraminidase and O-glycosidase (O-Gly), followed by immunoblotting with peroxidase-conjugated anti human IgG antibodies. (C) Detection of CD99L2 splice variants in bEnd.5 endothelioma cells and HUVEC by RT-PCR. (D) Immunofluorescence staining of bEnd.5 endothelioma cells with affinity purified antibodies against CD99L2 and negative control IgG from the respective preimmune serum (as indicated). First antibodies were detected with Cy3-conjugated secondary antibodies and visualized by fluorescence microscopy. Bar = 20 μm. (E) Immunoperoxidase staining of cryostat sections of mouse heart, kidney, and liver with affinity-purified antibodies against mouse CD99L2, mouse CD99, and control rabbit IgG from preimmune serum (as indicated). Tissue sections were incubated with first antibodies, followed by washing and incubation with secondary and tertiary reagent. Bar = 70 μm
In immunoblots of the mouse endothelioma cell line bEnd.5, antibodies against CD99L2 recognized a major band at 56 kd and a minor band at 40 kd (Figure 2A, lane 8). To determine whether these 2 bands could indeed represent the 2 splice variants, we performed RT-PCR experiments with total RNA from bEnd.5 cells. As shown in Figure 2C, 2 cDNA fragments were generated; the higher molecular weight form was the dominant one. The large splice variant was also the dominant one in HUVEC, besides 2 minor smaller variants (Figure 2C). Sequencing revealed that these products originated from the known splice variants. Thus, endothelial cells express mainly the larger splice variant of CD99L2 and only very small amounts of the smaller ones.
To examine the subcellular distribution of CD99L2, unfixed bEnd.5 endothelioma cells were incubated at 4°C with anti-CD99L2 antibodies, washed, fixed, and then stained with secondary antibodies. CD99L2 staining was found to be concentrated at cell-cell contacts of cultured endothelial cells, although it was also found on the entire surface of the cells (Figure 2D). On cryosections of various tissues such as heart, kidney, and liver, antibodies against mouse CD99L2 stained endothelial cells (Figure 2E) similarly to antibodies against CD99, which were used for controls.
CD99L2 is expressed on leukocytes
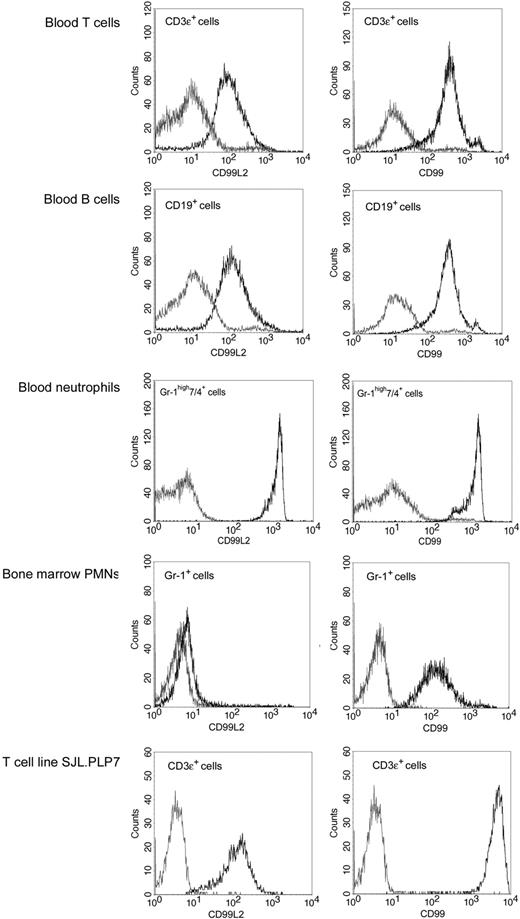
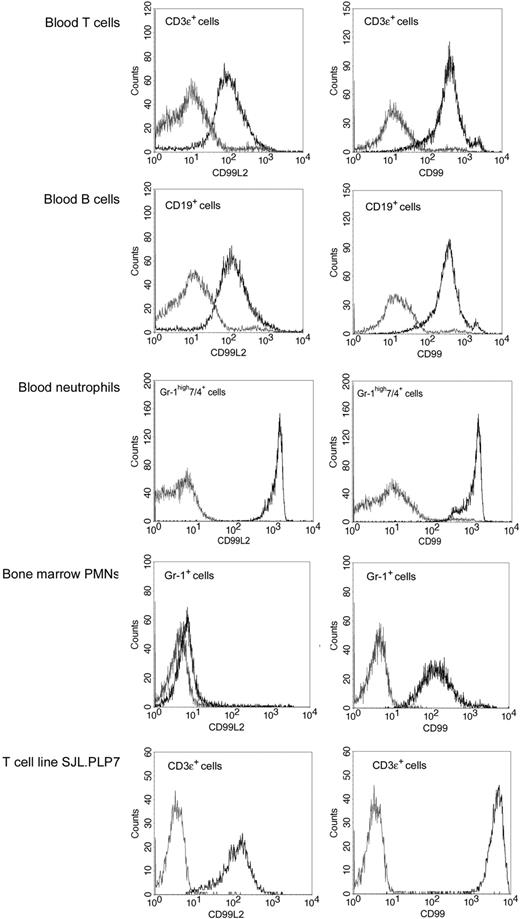
Cell-surface expression of CD99L2 on mouse leukocytes was analyzed by flow cytometry. T and B cells as well as neutrophils from peripheral blood were strongly positive for CD99L2, and the expression levels were comparable with those of CD99 (Figure 3). Analyzing leukocytes that we routinely use in in vitro transendothelial migration assays, we found that the T cell line SJL.PLP7 expressed CD99L2 at levels similar to those of primary lymphocytes, whereas bone marrow-derived neutrophils expressed almost undetectable levels of CD99L2 and well detectable levels of CD99. The drastic difference between the CD99L2-expression levels on peripheral and bone marrow-derived neutrophils makes CD99L2 one of very few antigens induced with neutrophil maturation.
Expression of CD99L2 on leukocytes and comparison with CD99. Detection of CD99L2 (left panels) and CD99 (right panels) primary T cells, B cells, and neutrophils isolated from blood and on neutrophils derived from bone marrow and on the T cell line SJL.PLP7 was analyzed by flow cytometry. Binding of affinity purified anti-CD99L2 or anti-CD99 antibodies (black lines) and control IgG (gray lines).
Expression of CD99L2 on leukocytes and comparison with CD99. Detection of CD99L2 (left panels) and CD99 (right panels) primary T cells, B cells, and neutrophils isolated from blood and on neutrophils derived from bone marrow and on the T cell line SJL.PLP7 was analyzed by flow cytometry. Binding of affinity purified anti-CD99L2 or anti-CD99 antibodies (black lines) and control IgG (gray lines).
CD99L2 supports cell aggregation of transfected cells
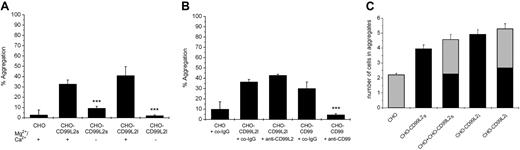
CD99 has been described to support homotypic cell aggregation, as was demonstrated for L cells transfected with human CD9918 and for CHO cells transfected with mouse CD99.19 To test whether CD99L2 would be able to support cell aggregation, we stably transfected CHO cells with either CD99L2L or CD99 L2S. As shown in Figure 4A, each of the 2 splice variants of CD99L2 enabled CHO cells to aggregate, and this homotypic cell aggregation required the presence of divalent cations, because it was strongly reduced in the absence of Mg2+ and Ca2+. We were surprised to find that, in clear contrast to CD99, antibodies against CD99L2 could not block CD99L2-dependent cell aggregation, whereas anti-CD99 antibodies, as we showed before, efficiently blocked aggregation of CD99-transfected cells (Figure 4B). Thus, either our anti-CD99L2 antibodies were not able to block a potential adhesive function of CD99L2, or CD99L2 was stimulating the adhesive function of other adhesion molecules.
CD99L2 supports aggregation of CD99L2-transfected CHO cells. (A) Mock-transfected CHO cells (CHO) or CHO cells stably transfected with CD99L2s (CHO-CD99L2S) or CD99L2L (CHO-CD99L2L) were allowed to aggregate in the presence (+) or in the absence (−) of Ca+2/Mg2+ as described under “Materials and methods.” ***, P < .001 CHO-CD99L2s with Mg2+/Ca2+ versus CHO-CD99L2s without Mg2+/Ca2+; ***P < .001 CHO-CD99L2l with Mg2+/Ca2+ versus CHO-CD99L2l without Mg2+/Ca2+. (B) Mock-transfected CHO cells (CHO) or CHO cells transfected with CD99L2L (CHO-CD99L2L) or CD99 (CHO-CD99) were allowed to aggregate in the presence of 30 μg/mL of the following antibodies: control antibodies (co-IgG), affinity-purified antibodies against CD99L2 (anti-CD99L2), or CD99 (anti-CD99) as indicated. ***P < .001 CHO-CD99 + anti-CD99 versus CHO-CD99 + co-IgG. (C) Mock-transfected CHO cells (CHO) or CHO cells stably transfected with CD99L2s (CHO-CD99L2S; labeled with Cell Tracker Green) or CD99L2L (CHO-CD99L2L; labeled with Cell Tracker Green) or mixed populations of mock-transfected or transfected cells (as indicated) were allowed to aggregate. Cell aggregates were analyzed by immunofluorescence microscopy and the number of mock-transfected cells ■ or transfected cells ■ per aggregate were counted. Statistical analysis was done by Student t test. Each panel is representative of at least 3 experiments; each measurement was done in triplicate.
CD99L2 supports aggregation of CD99L2-transfected CHO cells. (A) Mock-transfected CHO cells (CHO) or CHO cells stably transfected with CD99L2s (CHO-CD99L2S) or CD99L2L (CHO-CD99L2L) were allowed to aggregate in the presence (+) or in the absence (−) of Ca+2/Mg2+ as described under “Materials and methods.” ***, P < .001 CHO-CD99L2s with Mg2+/Ca2+ versus CHO-CD99L2s without Mg2+/Ca2+; ***P < .001 CHO-CD99L2l with Mg2+/Ca2+ versus CHO-CD99L2l without Mg2+/Ca2+. (B) Mock-transfected CHO cells (CHO) or CHO cells transfected with CD99L2L (CHO-CD99L2L) or CD99 (CHO-CD99) were allowed to aggregate in the presence of 30 μg/mL of the following antibodies: control antibodies (co-IgG), affinity-purified antibodies against CD99L2 (anti-CD99L2), or CD99 (anti-CD99) as indicated. ***P < .001 CHO-CD99 + anti-CD99 versus CHO-CD99 + co-IgG. (C) Mock-transfected CHO cells (CHO) or CHO cells stably transfected with CD99L2s (CHO-CD99L2S; labeled with Cell Tracker Green) or CD99L2L (CHO-CD99L2L; labeled with Cell Tracker Green) or mixed populations of mock-transfected or transfected cells (as indicated) were allowed to aggregate. Cell aggregates were analyzed by immunofluorescence microscopy and the number of mock-transfected cells ■ or transfected cells ■ per aggregate were counted. Statistical analysis was done by Student t test. Each panel is representative of at least 3 experiments; each measurement was done in triplicate.
It is unlikely that cell aggregation was dependent on homophilic CD99L2 interactions, because mixed populations of CD99L2-transfected and mock-transfected CHO cells aggregated as well as the same number of CD99L2-transfected cells. Aggregates of mixed cell populations contained 50% transfected and mock-transfected cells, respectively (Figure 4C). In agreement with this, we could not pull down CD99L2 from lysates of transfected cells with CD99L2-Fc (data not shown).
CD99L2, in contrast to CD99, is not required for lymphocyte extravasation
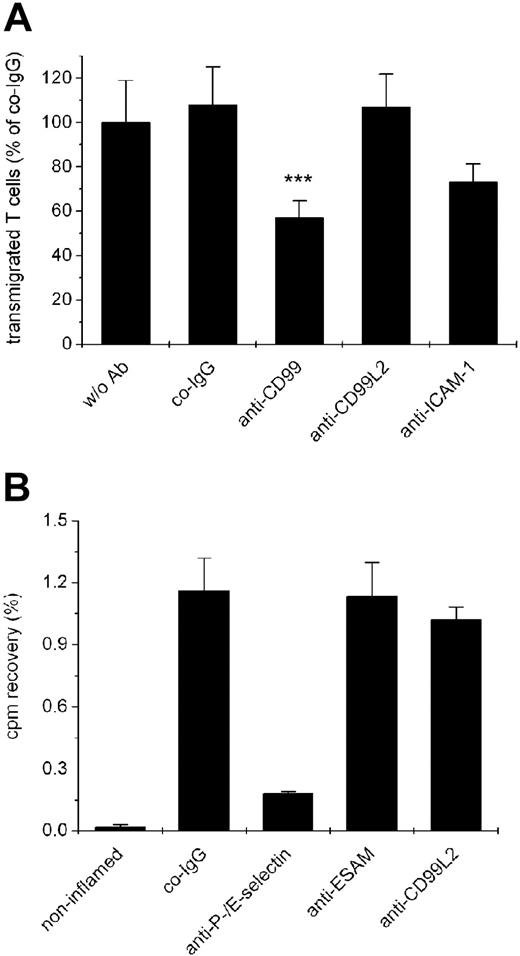
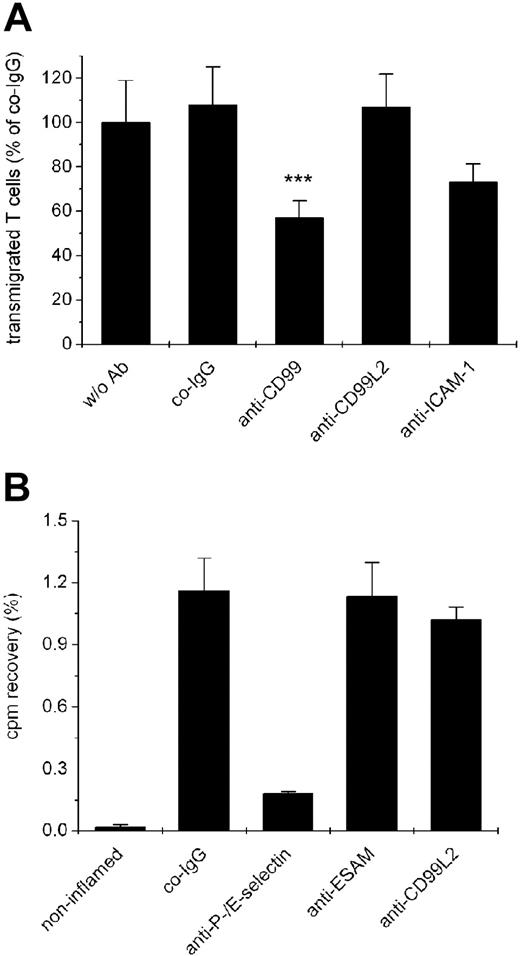
We have recently found that mouse CD99 is required for the migration of T cells through the monolayer of endothelial cells, as was shown by blocking transmigration of SJL/PLP7 T cells through the monolayer of bEnd.5 endothelial cells with anti-CD99 antibodies.19 Because both cell types express CD99L2 (as described above), we tested anti-CD99L2 antibodies in this assay. As shown in Figure 5A, no inhibitory effect of the antibodies was detected, whereas anti-CD99 and anti-ICAM-1 antibodies, as expected, efficiently inhibited T cell transmigration. The assays were performed for 30 minutes with the antibodies present during the assay and in the presence of the chemoattractant SDF-1 in the lower chamber. Comparable results were obtained when transmigration was allowed for 2 hours in the absence of a chemokine (data not shown).
Antibodies against CD99L2 do not affect lymphocyte transmigration in vitro and lymphocyte extravasation in vivo. (A) Transendothelial migration of SJL.PLP7 T lymphocytes is not affected by anti-CD99L2 antibodies. SJL.PLP7 cells were allowed to transmigrate through a monolayer of bEnd.5 cells grown on transwell filters for 30 minutes in the presence of 100 ng/mL SDF-1 in the lower chamber. Endothelial cells were incubated for 30 minutes before the assay either without antibodies (w/o Ab) or with preimmune control IgG (co-IgG), affinity purified anti-CD99 IgG (anti-CD99), affinity purified anti-CD99L2 IgG (anti-CD99L2), or a monoclonal antibody against ICAM-1 (anti-ICAM-1). Antibodies (each 30 μg/mL) remained present during the assay. Each panel is representative of at least 3 experiments; each measurement was done in triplicate. ***P < .001 anti-CD99 versus co-IgG. (B) Migration of lymphocytes into inflamed skin is not affected by anti-CD99L2. Radiolabeled in vivo-activated T cells were injected intravenously into hapten-challenged mice together with (50 μg each) control IgG from preimmune serum (co-IgG), or monoclonal antibodies against P-selectin and E-selectin (anti–P-/E-selectin), affinity-purified antibodies against ESAM (anti-ESAM), or affinity-purified antibodies against CD99L2 (anti-CD99L2). The bar on the left represents radioactivity accumulating in the noninflamed ear. Statistical analysis was done by Student t test. Results are representative for 3 similar experiments; for each determination, 4 mice were analyzed. Numbers on the left refer to the percentage of injected cells that were found in the analyzed ear.
Antibodies against CD99L2 do not affect lymphocyte transmigration in vitro and lymphocyte extravasation in vivo. (A) Transendothelial migration of SJL.PLP7 T lymphocytes is not affected by anti-CD99L2 antibodies. SJL.PLP7 cells were allowed to transmigrate through a monolayer of bEnd.5 cells grown on transwell filters for 30 minutes in the presence of 100 ng/mL SDF-1 in the lower chamber. Endothelial cells were incubated for 30 minutes before the assay either without antibodies (w/o Ab) or with preimmune control IgG (co-IgG), affinity purified anti-CD99 IgG (anti-CD99), affinity purified anti-CD99L2 IgG (anti-CD99L2), or a monoclonal antibody against ICAM-1 (anti-ICAM-1). Antibodies (each 30 μg/mL) remained present during the assay. Each panel is representative of at least 3 experiments; each measurement was done in triplicate. ***P < .001 anti-CD99 versus co-IgG. (B) Migration of lymphocytes into inflamed skin is not affected by anti-CD99L2. Radiolabeled in vivo-activated T cells were injected intravenously into hapten-challenged mice together with (50 μg each) control IgG from preimmune serum (co-IgG), or monoclonal antibodies against P-selectin and E-selectin (anti–P-/E-selectin), affinity-purified antibodies against ESAM (anti-ESAM), or affinity-purified antibodies against CD99L2 (anti-CD99L2). The bar on the left represents radioactivity accumulating in the noninflamed ear. Statistical analysis was done by Student t test. Results are representative for 3 similar experiments; for each determination, 4 mice were analyzed. Numbers on the left refer to the percentage of injected cells that were found in the analyzed ear.
Despite this lack of inhibition, we tested whether these antibodies would affect the recruitment of T cells into inflamed areas of the mouse skin in a typical delayed type hypersensitivity (DTH) model. In vivo activated T cells were isolated from lymph nodes of dinitrofluorobenzene (DNFB)-treated mice, labeled with 51Cr, and injected intravenously into mice with a DNFB-elicited DTH reaction in the right ear. Affinity-purified antibodies against CD99L2, negative control antibodies from preimmune serum or from a serum against ESAM, and positive control antibodies against E- and P-selectin were coinjected with the T cells. Mice were killed 15 hours later, and radioactivity in the right ear was determined. As shown in Figure 5B, T cell recruitment was only inhibited with antibodies against E- and P-selectin, whereas the antibodies against CD99L2 had no effect. Thus, CD99L2 seems to differ considerably from CD99, and only antibodies against the latter interfere with lymphocyte extravasation.
CD99L2 as well as CD99 are involved in the transendothelial migration of neutrophils in vitro
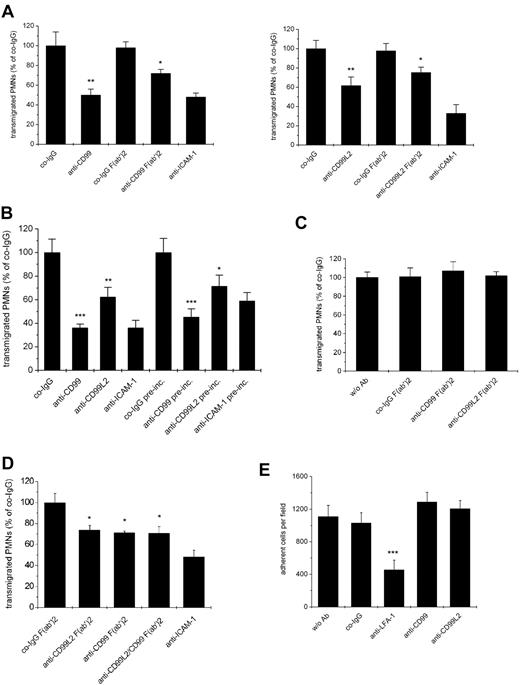
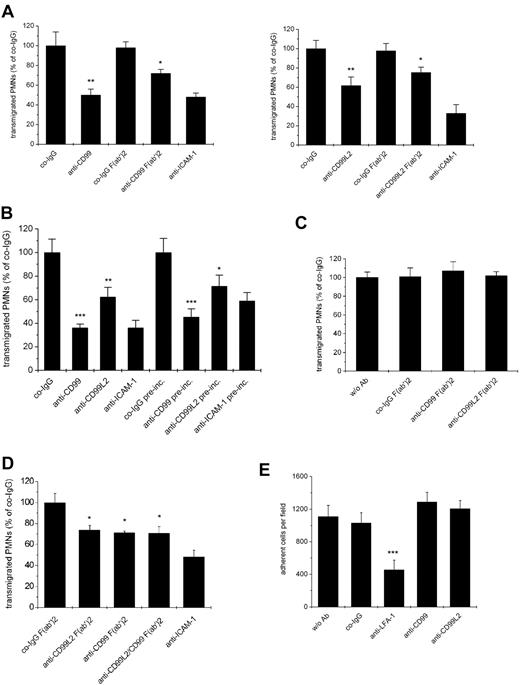
Because human CD99 had been reported to be involved in the transmigration of human monocytes,18 we tested whether mouse CD99 or CD99L2 are relevant for the transendothelial migration of mouse myeloid cells. Because of the low yields of mouse neutrophils that can be obtained from blood, we used neutrophils isolated from bone marrow, although they expressed only low amounts of CD99 and almost undetectable levels of CD99L2. Remarkably, and in contrast to the situation with lymphocytes, antibodies against each antigen, CD99 as well as CD99L2, clearly inhibited transmigration of neutrophils (Figure 6A). A similar result was found for F(ab′)2 fragments of both antibody preparations, excluding potential problems with myeloid Fc-receptors (Figure 6A). This establishes for the first time CD99 and CD99L2 as molecules involved in the transendothelial migration of mouse neutrophils. In addition, it demonstrates that our anti CD99L2 antibodies are indeed able to block a function of this antigen.
Antibodies against CD99L2 and CD99 block transendothelial migration of neutrophils in vitro. (A) Antibodies against CD99 or CD99L2 inhibit transendothelial migration of PMNs through a bEnd.5 monolayer. Bone-marrow derived PMNs were allowed to transmigrate for 30 minutes through a monolayer of bEnd.5 cells grown on transwell filters in the presence of 100 ng/mL KC in the lower chamber. Endothelial cells were incubated for 30 minute with 30 μg/mL of antibody before the start of the assay as indicated. Left panel: with preimmune control IgG (co-IgG) or affinity purified anti-CD99 IgG (anti-CD99) or F(ab′)2 fragments of control IgG (co-IgG F(ab′)2) or anti-CD99 IgG (anti-CD99 F(ab′)2) or a monoclonal antibody against ICAM-1 (anti-ICAM-1). **, P < .01 anti-CD99 versus co-IgG; *, P < .05 anti-CD99 F(ab′)2 versus co-IgG F(ab′)2. Right panel: with preimmune control IgG (co-IgG) or affinity purified anti-CD99L2 IgG (anti-CD99L2) or F(ab′)2 fragments of control IgG (co-IgG F(ab′)2) or anti-CD99L2 IgG (anti-CD99L2 F(ab′)2) or a monoclonal antibody against ICAM-1 (anti-ICAM-1). **, P < .01 anti-CD99 versus co-IgG; *, P < .05 anti-CD99 F(ab′)2 versus co-IgG F(ab′)2. Antibodies remained present during the assay. (B) After preincubation of the endothelial cells with the indicated antibodies, the antibodies either remained present during the assay (first 4 bars) or were washed away before adding the PMNs (indicated by “pre-inc.” underneath the last 4 bars). ***, P < .001 anti-CD99 versus co-IgG; **, P < .01 anti-CD99L2 versus co-IgG; ***, P < .001 anti-CD99 pre-inc. versus co-IgG pre-inc. *, P < .05 anti-CD99L2 pre-inc. versus co-IgG pre-inc. (C) PMNs were preincubated with medium without antibody (w/o Ab) or with F(ab′)2 fragments of control IgG (co-IgG F(ab′)2), or anti-CD99 IgG (anti-CD99 F(ab′)2) or anti-CD99L2 IgG (anti-CD99L2 F(ab′)2). Antibodies were washed away before adding the PMNs into the assay. Each panel is representative of at least 3 experiments; each measurement was done in triplicate. (D) No additive effect of anti-CD99 and anti-CD99L2 F(ab′)2-fragments: experiments were done as in (A), endothelial cells were preincubated with F(ab′)2 fragments of control IgG (co-IgG F(ab′)2), anti-CD99L2 F(ab′)2 (anti-CD99L2 F(ab′)2), anti-CD99 F(ab′)2 (anti-CD99 F(ab′)2), or a mixture of anti CD99L2 and CD99 F(ab′)2 (anti CD99L2/CD99 F(ab′)2), or with monoclonal antibody against ICAM-1 (anti-ICAM-1). (E) Binding of PMNs to endothelial cells is not affected by antibodies against CD99 or CD99L2. Bone-marrow derived PMNs were allowed to adhere to a monolayer of bEnd.5 cells in the absence of antibody (w/o Ab) or in the presence of control antibodies (co-IgG) or antibodies against LFA-1 (anti-LFA-1), affinity-purified antibodies against CD99 (anti-CD99) or CD99L2 (anti-CD99L2). ***, P < .001 anti-LFA-1 vs. co-IgG. Statisitical analysis was done by Student t test. Each panel is representative of at least 3 experiments; each measurement was done in triplicate.
Antibodies against CD99L2 and CD99 block transendothelial migration of neutrophils in vitro. (A) Antibodies against CD99 or CD99L2 inhibit transendothelial migration of PMNs through a bEnd.5 monolayer. Bone-marrow derived PMNs were allowed to transmigrate for 30 minutes through a monolayer of bEnd.5 cells grown on transwell filters in the presence of 100 ng/mL KC in the lower chamber. Endothelial cells were incubated for 30 minute with 30 μg/mL of antibody before the start of the assay as indicated. Left panel: with preimmune control IgG (co-IgG) or affinity purified anti-CD99 IgG (anti-CD99) or F(ab′)2 fragments of control IgG (co-IgG F(ab′)2) or anti-CD99 IgG (anti-CD99 F(ab′)2) or a monoclonal antibody against ICAM-1 (anti-ICAM-1). **, P < .01 anti-CD99 versus co-IgG; *, P < .05 anti-CD99 F(ab′)2 versus co-IgG F(ab′)2. Right panel: with preimmune control IgG (co-IgG) or affinity purified anti-CD99L2 IgG (anti-CD99L2) or F(ab′)2 fragments of control IgG (co-IgG F(ab′)2) or anti-CD99L2 IgG (anti-CD99L2 F(ab′)2) or a monoclonal antibody against ICAM-1 (anti-ICAM-1). **, P < .01 anti-CD99 versus co-IgG; *, P < .05 anti-CD99 F(ab′)2 versus co-IgG F(ab′)2. Antibodies remained present during the assay. (B) After preincubation of the endothelial cells with the indicated antibodies, the antibodies either remained present during the assay (first 4 bars) or were washed away before adding the PMNs (indicated by “pre-inc.” underneath the last 4 bars). ***, P < .001 anti-CD99 versus co-IgG; **, P < .01 anti-CD99L2 versus co-IgG; ***, P < .001 anti-CD99 pre-inc. versus co-IgG pre-inc. *, P < .05 anti-CD99L2 pre-inc. versus co-IgG pre-inc. (C) PMNs were preincubated with medium without antibody (w/o Ab) or with F(ab′)2 fragments of control IgG (co-IgG F(ab′)2), or anti-CD99 IgG (anti-CD99 F(ab′)2) or anti-CD99L2 IgG (anti-CD99L2 F(ab′)2). Antibodies were washed away before adding the PMNs into the assay. Each panel is representative of at least 3 experiments; each measurement was done in triplicate. (D) No additive effect of anti-CD99 and anti-CD99L2 F(ab′)2-fragments: experiments were done as in (A), endothelial cells were preincubated with F(ab′)2 fragments of control IgG (co-IgG F(ab′)2), anti-CD99L2 F(ab′)2 (anti-CD99L2 F(ab′)2), anti-CD99 F(ab′)2 (anti-CD99 F(ab′)2), or a mixture of anti CD99L2 and CD99 F(ab′)2 (anti CD99L2/CD99 F(ab′)2), or with monoclonal antibody against ICAM-1 (anti-ICAM-1). (E) Binding of PMNs to endothelial cells is not affected by antibodies against CD99 or CD99L2. Bone-marrow derived PMNs were allowed to adhere to a monolayer of bEnd.5 cells in the absence of antibody (w/o Ab) or in the presence of control antibodies (co-IgG) or antibodies against LFA-1 (anti-LFA-1), affinity-purified antibodies against CD99 (anti-CD99) or CD99L2 (anti-CD99L2). ***, P < .001 anti-LFA-1 vs. co-IgG. Statisitical analysis was done by Student t test. Each panel is representative of at least 3 experiments; each measurement was done in triplicate.
The low expression of CD99 and the hardly detectable expression of CD99L2 on bone marrow-derived neutrophils already suggested that the antibodies affected transmigration via binding to the endothelial antigens. To test this more directly, we preincubated bEnd.5 cells with either anti-CD99 or anti-CD99L2 antibodies and washed them away before the assay. We found that this treatment reduced neutrophil transmigration almost as efficiently as when antibodies were still present during the assay (Figure 6B), demonstrating that indeed each of the 2 antigens acted on the endothelial side. Preincubating only neutrophils with antibodies before the assay did not yield conclusive results, because the test antibodies as well as negative control antibodies from the preimmune sera inhibited transmigration, possibly as a result of nonspecifically activating neutrophils via Fc-receptors. To avoid this problem, we preincubated neutrophils with F(ab′)2 fragments of anti-CD99, anti-CD99L2, and preimmune antibodies before the assay. As shown in Figure 6C, neither anti-CD99 nor anti-CD99L2 F(ab′)2-fragments inhibited neutrophil transmigration. This had been expected for CD99L2, because this antigen had not been detected in significant amounts on neutrophils. However, it is surprising that CD99 on neutrophils was not involved in neutrophil transmigration, because the transmigration of lymphocytes requires CD99 on the lymphocyte side as well as on the endothelial surface.19
To determine whether CD99 and CD99L2 act in independent pathways, we tested whether F(ab′)2-fragments of antibodies against each antigen would have additive inhibitory effects. As shown in Figure 6D, both types of F(ab′)2-fragments together had no stronger inhibitory effect than F(ab′)2-fragments against each antigen alone. We conclude that the CD99-independent extravasation of neutrophils was also CD99L2-independent.
To distinguish whether either of the 2 antigens would participate in neutrophil transmigration via mediating adhesion of neutrophils to endothelial cells or via participating in the transendothelial migration step, we tested whether the antibodies would inhibit adhesion of neutrophils to bEnd.5 cells. No such inhibitory effect was found (Figure 6E). We conclude that CD99 as well as CD99L2 on endothelial cells are involved in the migration of neutrophils through endothelial-cell layers.
CD99 and CD99L2 participate in the migration of neutrophils into inflamed peritoneum
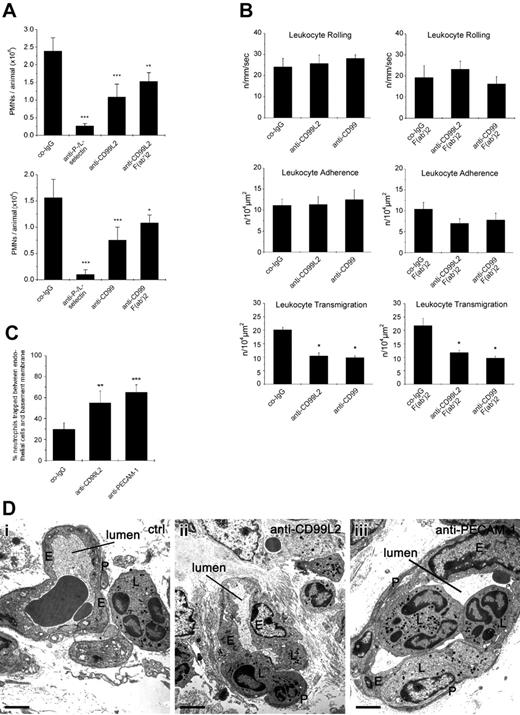
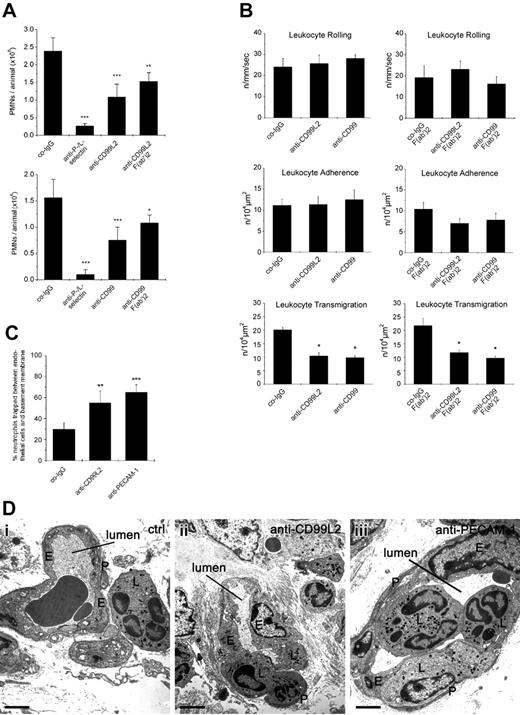
Chemically induced peritonitis is a well-characterized model to study neutrophil recruitment into sites of acute inflammation in vivo. We tested whether i.v. injection of either anti-CD99 or anti-CD99L2 antibodies immediately before intraperitoneal injection of thioglycollate would affect the recruitment of neutrophils into the peritoneum within a 4-hour period after stimulation. We found that antibodies against CD99 reduced neutrophil accumulation by 59% (±12%), whereas anti-CD99L2 antibodies inhibited by 69% (±5%) compared with negative control antibodies from the preimmune sera (Figure 7A). Combined treatment with anti L- and anti P-selectin antibodies blocked neutrophil recruitment by more than 85%. Injection of PBS without antibodies gave results similar to those of negative control antibodies (data not shown). To exclude the possibility that interactions with Fc-receptors or complement would interfere with our results, we also tested F(ab′)2-fragments. As shown in Figure 7A, F(ab′)2-fragments of anti-CD99 as well as of CD99L2 antibodies reduced neutrophil recruitment into inflamed peritoneum significantly, although the inhibitory effect was less pronounced compared with the decrease observed with intact antibodies.
Antibodies against CD99L2 and CD99 inhibit neutrophil extravasation in vivo by blocking diapedesis. (A) Peritonitis assay: mice were intravenously injected with 50 μg antibody: preimmune control IgG (co-IgG) for negative controls, or with monoclonal antibodies against P-selectin and L-selectin (anti-P-/L-selectin) for positive controls, or with affinity purified anti-CD99L2 IgG (anti-CD99L2) or F(ab′)2 fragments of anti-CD99L2 IgG (anti-CD99L2 F(ab′)2) (upper panel) or with affinity purified anti-CD99 IgG (anti-CD99) or F(ab′)2 fragments of anti-CD99 IgG (anti-CD99 F(ab′)2) (bottom panel) in PBS immediately followed by intraperitoneal administration of thioglycollate. Peritoneal leukocytes were removed at 4 hours after stimulation, and neutrophil counts were determined. Results represent 3 independent experiments, for each determination of each experiment 5 mice were analyzed. Left panel, ***, P < .001 anti-P-/L-selectin versus co-IgG; ***, P < .001 anti-CD99L2 versus co-IgG; **, P < .01 anti-CD99L2 F(ab′)2 versus co-IgG. Right panel: ***, P < .001 anti-P-/L-selectin versus co-IgG; ***, P < .001 anti-CD99L2 versus co-IgG; *, P < .05 anti-CD99L2 F(ab′)2 versus co-IgG. Statistical analysis was done by Student t test. (B) Intravital microscopy of cremaster muscle venules: mice were intrascrotally stimulated with IL-1β and intravenously injected with 50 μg antibody of preimmune control IgG (co-IgG), or affinity-purified anti-CD99L2 IgG (anti-CD99L2) or affinity-purified anti-CD99 IgG (anti-CD99) (left panels) or with 75 μg of control-IgG-F(ab′)2-fragments (co-IgG-F(ab′)2), or anti-CD99L2-F(ab′)2 or anti-CD99-F(ab′)2 (as indicated) (right panels). Four hours later, the cremaster muscle was surgically prepared, and the number of rolling leukocytes per second per millimeter of vessel diameter, the number of adherent leukocytes per 104 μm2 of venule surface area, and the number of extravasated leukocytes from cremasteric venules per 104 μm2 tissue area were determined by intravital near-infrared reflected light oblique transillumination microscopy. *, P < .05 versus co-IgG, statistical analysis was done by ANOVA. (C) Proportion of neutrophils trapped between endothelial cells and the basement membrane/pericyte layer in IL-1β treated cremaster muscle. Analysis was done by transmission electron microscopy. Animals were treated with preimmune control antibodies (co-IgG), anti-CD99L2 antibodies (anti-CD99L2), or with a mixture of 2 monoclonal antibodies against mouse PECAM-1 (anti-PECAM-1). The graph shows the number of neutrophils found between the endothelium and the underlying layer of basement membranes and pericytes, given as percentage of leukocytes that had passed the endothelial cell contacts. For each determination 30 to 52 randomly selected vessel segments were analyzed. (D) Electron micrographs of vessel segments of IL-1β activated cremaster muscle of mice treated with control antibody (ctrl: a), anti-CD99L2 antibodies (anti-CD99L2: b), or anti-PECAM-1 mAbs (anti-PECAM-1: c). E, endothelium; L, leukocyte; P, pericyte. The lumen of the vessel is indicated. Bar: 2 μm.
Antibodies against CD99L2 and CD99 inhibit neutrophil extravasation in vivo by blocking diapedesis. (A) Peritonitis assay: mice were intravenously injected with 50 μg antibody: preimmune control IgG (co-IgG) for negative controls, or with monoclonal antibodies against P-selectin and L-selectin (anti-P-/L-selectin) for positive controls, or with affinity purified anti-CD99L2 IgG (anti-CD99L2) or F(ab′)2 fragments of anti-CD99L2 IgG (anti-CD99L2 F(ab′)2) (upper panel) or with affinity purified anti-CD99 IgG (anti-CD99) or F(ab′)2 fragments of anti-CD99 IgG (anti-CD99 F(ab′)2) (bottom panel) in PBS immediately followed by intraperitoneal administration of thioglycollate. Peritoneal leukocytes were removed at 4 hours after stimulation, and neutrophil counts were determined. Results represent 3 independent experiments, for each determination of each experiment 5 mice were analyzed. Left panel, ***, P < .001 anti-P-/L-selectin versus co-IgG; ***, P < .001 anti-CD99L2 versus co-IgG; **, P < .01 anti-CD99L2 F(ab′)2 versus co-IgG. Right panel: ***, P < .001 anti-P-/L-selectin versus co-IgG; ***, P < .001 anti-CD99L2 versus co-IgG; *, P < .05 anti-CD99L2 F(ab′)2 versus co-IgG. Statistical analysis was done by Student t test. (B) Intravital microscopy of cremaster muscle venules: mice were intrascrotally stimulated with IL-1β and intravenously injected with 50 μg antibody of preimmune control IgG (co-IgG), or affinity-purified anti-CD99L2 IgG (anti-CD99L2) or affinity-purified anti-CD99 IgG (anti-CD99) (left panels) or with 75 μg of control-IgG-F(ab′)2-fragments (co-IgG-F(ab′)2), or anti-CD99L2-F(ab′)2 or anti-CD99-F(ab′)2 (as indicated) (right panels). Four hours later, the cremaster muscle was surgically prepared, and the number of rolling leukocytes per second per millimeter of vessel diameter, the number of adherent leukocytes per 104 μm2 of venule surface area, and the number of extravasated leukocytes from cremasteric venules per 104 μm2 tissue area were determined by intravital near-infrared reflected light oblique transillumination microscopy. *, P < .05 versus co-IgG, statistical analysis was done by ANOVA. (C) Proportion of neutrophils trapped between endothelial cells and the basement membrane/pericyte layer in IL-1β treated cremaster muscle. Analysis was done by transmission electron microscopy. Animals were treated with preimmune control antibodies (co-IgG), anti-CD99L2 antibodies (anti-CD99L2), or with a mixture of 2 monoclonal antibodies against mouse PECAM-1 (anti-PECAM-1). The graph shows the number of neutrophils found between the endothelium and the underlying layer of basement membranes and pericytes, given as percentage of leukocytes that had passed the endothelial cell contacts. For each determination 30 to 52 randomly selected vessel segments were analyzed. (D) Electron micrographs of vessel segments of IL-1β activated cremaster muscle of mice treated with control antibody (ctrl: a), anti-CD99L2 antibodies (anti-CD99L2: b), or anti-PECAM-1 mAbs (anti-PECAM-1: c). E, endothelium; L, leukocyte; P, pericyte. The lumen of the vessel is indicated. Bar: 2 μm.
Antibodies against CD99 or CD99L2 inhibit neutrophil extravasation in vivo at the level of transmigration
To clarify in vivo at which step CD99L2 and CD99 are involved during the process of leukocyte extravasation, we visualized the process in the cremaster muscle using intravital near-infrared reflected light oblique transillumination microscopy.30 Under the experimental conditions used, the vast majority (more than 80%) of extravasating leukocytes are neutrophils.15 Mice were intravenously injected with antibodies and stimulated intrascrotally with IL-1β for 4 hours before microscopic analysis. As can be seen in Figure 7B and Figure S2, neither anti-CD99 nor anti-CD99L2 antibodies affected rolling or adhesion of leukocytes in the observed cremaster venules. Instead, antibodies against both antigens significantly reduced the number of extravasated neutrophils accumulating in the observation area. Similar results were obtained with F(ab′)2-fragments (Figure 7B). Thus, CD99 as well as CD99L2 are involved in vivo in the diapedesis of neutrophils.
To determine at which step of the diapedesis process the antibodies against CD99L2 arrest leukocytes, the cremaster tissue was analyzed by electron microscopy. Animals treated with antibodies against CD99L2 or against PECAM-1 had significantly larger numbers of leukocytes trapped between the endothelium and the basement membrane/pericyte layer than animals treated with control antibodies (Figure 7C,D). Independent of the type of antibody used, the number of leukocytes trapped between endothelial cells (category B) was very small (3%—5% of all extravasating cells: B/B+C+D) with no significant changes between the different groups.
Discussion
CD99L2 was recently cloned as a CD99-related gene coding for a protein of unknown function, which is widely distributed in various tissues.20 Here we have identified CD99L2 as a novel cell surface protein on endothelial cells and leukocytes that participates in vivo in the recruitment of neutrophils into inflamed tissue.
Comparing the function of CD99L2 with that of CD99 in more detail revealed important similarities and striking differences. There are 4 important characteristics that both proteins share. First, both proteins are structurally related, with 32% amino acid identity between mouse CD99 and CD99L2. They are both type 1 transmembrane proteins with extracellular domains only slightly larger than 100 amino acids, rich inO-linked carbohydrates, with no resemblance to any known protein family. Second, both proteins are expressed on endothelium as well as on lymphocytes and neutrophil granulocytes. Third, both proteins support cell aggregation upon ectopic expression in CHO cells. Fourth and most importantly, both proteins participate in the diapedesis process and not in the docking of leukocytes to the vessel wall. Although this was already anticipated for CD99 from in vitro transmigration assays,18,19,32 our results demonstrate this for the first time in vivo, for CD99 as well as for CD99L2. Thus, CD99 and CD99L2 represent a new class of membrane proteins, clearly distinct from the Ig-SF, that participate in leukocyte diapedesis.
It is noteworthy that we could demonstrate by electron microscopy that anti-CD99L2 antibodies trap neutrophils between the basal side of the endothelium and the basement membrane/pericyte layer similarly to anti-PECAM-1 antibodies. This confirms other studies for PECAM-133,34 and establishes CD99L2 as the second endothelial cell contact protein for which it has been determined in vivo at which step during diapedesis it is required.
Despite the many similarities, CD99 and CD99L2 differ in important aspects. Most importantly, they participate in the extravasation of different repertoires of leukocytes. Whereas both proteins mediate the extravasation of neutrophils, only CD99 is relevant for the extravasation of lymphocytes, despite strong expression of CD99L2 on lymphocytes. The possibility that CD99L2 might even participate in lymphocyte extravasation and that our antibodies are merely unable to block this process is unlikely, although cannot be ruled out.
It has been suggested that CD99 may participate in lymphocyte diapedesis via homophilic interactions. This is based on the findings that transmigration required CD99 on lymphocytes and on endothelial cells and that CD99 supports homotypic cell aggregation of transfected cells.19 Similar results have been demonstrated for human CD99 and its role in transmigration of monocytes.18 Obviously CD99L2 on lymphocytes is not able to function in the same way, because it is not involved in lymphocyte diapedesis although it is present on the surface of both cell types.
It is noteworthy that neutrophils seem to extravasate in a different way than lymphocytes with respect to the participation of CD99 and CD99L2. Both proteins on endothelial cells are involved in the transendothelial migration of neutrophils, because antibodies against each of the 2 proteins inhibited transmigration, when incubated with endothelial cells only. It is unlikely that endothelial CD99 or CD99L2 participated in diapedesis by homophilic binding to their respective counterpart on neutrophils because, first, CD99L2 was almost undetectable on bone marrow-derived neutrophils and CD99 was only weakly expressed. Second and more importantly, even if the weak expression levels of CD99 and CD99L2 on bone marrow-derived neutrophils would have been sufficient to serve as binding partners for endothelial CD99 or CD99L2, it would have been expected that incubating neutrophils with antibodies against CD99 or CD99L2 would have reduced transmigration. Because this was not the case, we conclude that endothelial CD99 and CD99L2 probably can mediate neutrophil transmigration independent of their respective counterparts on the neutrophil surface. However, we cannot exclude that, in addition, CD99 or CD99L2 on peripheral neutrophils could be involved in diapedesis, because both proteins are expressed at much higher levels on these cells than on bone marrow-derived neutrophils.
A role of CD99L2 in supporting cell interactions independent of homophilic adhesive interactions is also suggested by our cell aggregation assays with CD99L2-transfected CHO cells. Although CD99L2 promoted cell aggregation, this could not be blocked with antibodies, despite their ability to block the function of CD99L2 in diapedesis. Thus, it is uncertain whether the cell aggregation-promoting adhesion function of CD99L2 is involved in diapedesis. CD99L2 could trigger homotypic cell aggregation by stimulating other adhesion molecules or effects on the membrane underlying cytoskeleton. Although this is possible, we can exclude that anti-CD99 or anti-CD99L2 antibodies inhibit diapedesis by simply affecting adhesive mechanisms that mediate the binding of leukocytes to endothelial cells, because the antibodies had no effects in adhesion assays with lymphocytes (data not shown) or neutrophils (Figure 6) on endothelial cell monolayers.
It is remarkable that the anti-CD99 and anti-CD99L2 antibodies had no additive inhibitory effects on neutrophil transmigration. Thus, they do not act independently. Potential heterophilic binding of CD99 to CD99L2 in pull-down experiments, however, could not be demonstrated (not shown).
Further investigations are necessary to reveal the mechanisms that enable CD99 and CD99L2 to control and mediate leukocyte diapedesis. Whatever mechanism will be elucidated for CD99 and CD99L2, our present study establishes CD99L2 as a cell surface protein on endothelium that represents a novel target to block infiltration of neutrophils into inflamed tissue.
The online version of this manuscript contains a data supplement.
An Inside Blood analysis of this article appears at the front of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Birgit Kempe, Yeliz Donat, Ria Knittel, and Gabi Frommer-Kästle for excellent technical assistance.
Supported by funding through the Interdisziplinäres Klinisches Forschungszentrum (IZKF) Münster and the Max-Planck-Gesellschaft (to D.V.) and through the European Community (NoE MAIN 502935) (to F.K.).
Authorship
Contribution: M.G.B., B.P., A.G.K., A. K., K. W.-B., H.W., and S.M. performed, designed, and analyzed experiments; F.K. and D.V. initiated the study and designed and supervised the research project; M.G.B., and D.V. contributed to data analysis and interpretation and wrote the paper.
Conflict -of-interest disclosure: The authors declare no competing financial interests.
Contribution: Dietmar Vestweber, Max-Planck-Institute of Molecular Biomedicine, Röntgenstr. 20, D-48149 Münster, Germany; e-mail: vestweb@mpi-muenster.mpg.de.