Abstract
Cutaneous mast cells have important pathogenic roles in skin inflammation, but the signals regulating mast-cell numbers in healthy and inflamed skin are not fully understood. Mast-cell development depends on the receptor tyrosine kinase Kit as shown by a greater than 95% reduction of mast-cell numbers in hypomorphic (KitW/Wv) mutant mice that are widely used as a mast-cell deficiency model. Mast-cell numbers are normally very low in KitW/Wv mice, but numbers can strongly increase under inflammatory conditions. It remains elusive whether this inflammation-driven mast-cell accumulation is mediated by signals transmitted via the KitWv receptor or by other, Kit-independent stimuli. We show here, using viable Kit- null mice (KitW/W), that Kit is essential for mast-cell accumulation in phorbol-12-myristate-13-acetate (PMA)–treated, chronically inflamed skin. This increase in mast- cell numbers is strongly attenuated in KitW/Wv mice lacking mature lymphocytes (T, B, and natural killer [NK] cells). These data, together with reconstitution experiments, point at a role for lymphocytes in the regulation of mast-cell compartments under limiting Kit signaling. We conclude that inflammation-induced cutaneous mast-cell accumulation is dependent on Kit signaling strength, and, under limiting Kit signals, on cells of the adaptive immune system.
Introduction
Connective tissue mast cells reside in a variety of tissues, notably skin and peritoneal cavity (reviewed in Galli et al1 ). The exact hematopoietic pathways that fill and maintain mast cell compartments are still poorly understood. Mast cell–committed progenitors have been identified in fetal blood,2 adult bone marrow (BM),3,4 and spleen,5 and their existence suggests that mast cell commitment can precede tissue immigration. A crucial growth factor receptor that drives mast cell development in vivo is the receptor tyrosine kinase Kit. The importance of Kit is documented by the drastic reduction of mast cell numbers in mice with inactivating mutations in Kit or Kit ligand (stem cell factor [Scf]) genes6 (reviewed in Besmer,7 Galli et al,8 and Broudy9 ). In the skin, Kit signals can regulate mast cell numbers as shown by local mast cell proliferation and activation after subcutaneous administration of exogenous Scf.10,11 In contrast to steady state mast cell development, less information exists on the requirement for Kit signaling in mast cells under inflammatory conditions.
KitW/Wv mice have been widely used as an in vivo model for mast cell deficiency. These mice have only approximately 1% of normal mast cell numbers in their skin, and they lack peritoneal mast cells.6 KitW/Wv mice express the KitW protein together with the KitWv protein. The KitW protein lacks the transmembrane domain and thus cannot be expressed on the cell surface. In KitW/Wv mice, Kit expression is reduced to about 5% of wild-type levels.12 In addition to this low cell-surface expression, the function of the KitWv receptor is impaired because of a point mutation that attenuates its kinase activity.13
Skin mast cells are present constitutively (ie, in the absence of an immune response), but their numbers markedly increase under local inflammatory conditions. Despite impaired Kit expression and function, mast cell numbers can also increase strongly (10- to 100-fold) in the skin of KitW/Wv mice during the natural history of an idiopathic dermatitis14 or during phorbol-12-myristate-13-acetate (PMA)–induced chronic dermatitis.15
Mice homozygous for KitW alleles die within a few days after birth,16 a fact that has precluded analyses of mast cells in adult KitW/W mice. KitW/W mice suffer from severe anemia16 that is the cause of their lethality.17 Stimulation of erythropoiesis by transgenic overexpression of a human erythropoietin (EPO) gene is sufficient to generate viable KitW/W mice.17 In these adult KitW/WTg(EPO) mice (referred to as KitW/W) the transgene has no salvage effects on hematopoietic cells outside the erythrocyte lineage, including stem/progenitor cells12,17 and mast cells (see Figure 2).
The fact that chronic inflammation induces mast cell accumulation even in KitW/Wv mice raises the possibility that Kit signaling is not essential under inflammatory conditions. Alternatively, the residual Kit expression/signaling in KitW/Wv mast cells is sufficient to increase mast cell numbers. To solve this question, we have challenged the skin of adult KitW/W mice by PMA to induce a chronic irritative dermatitis. In contrast to KitW/Wv mice that showed a massive mast cell accumulation, the skin of KitW/W mice remained free of mast cells. To address the role of lymphocytes during this inflammation-induced mast cell accumulation, we have also generated and analyzed new compound mutants. In these triple mutant mice, termed R−γ−KitW/Wv, the mast cell deficiency of KitW/Wv mice was combined with lack of all lymphocytes owing to mutations in the recombination activating gene (Rag-2) and the common cytokine receptor gamma chain (γc) gene. Of note, inflammation-driven mast cell expansion was severely impaired in these compound R−γ−KitW/Wv mutants. Adoptive transfer experiments suggest that this defect in mast cell reconstitution was caused by the absence of lymphocytes rather than lack of γc, a receptor subunit invoked in mast cell growth. Hence, in this report, we provide new insights into the cellular and molecular regulation of mast cell numbers in the skin.
Materials and methods
Mice
WBKitW/+ (strain, WB), C57BL/6KitWv/+ (strain, C57BL/6) mice (Japan-SLC, Shizuoka, Japan) and WBB6F1KitW/Wv (strain, F1 of WB × C57BL/6) mice were continuously bred in our animal facility. Parental mouse strains for Rag-2−/−γc−KitW/Wv (termed R−γ−KitW/Wv) triple mutant mice were generated by crossing Rag-2−/−18 γc−19 double-mutant mice (strain, mixed C57BL/6 and C57BL/10 background; Taconic, Germantown, NY) to WBKitW/+ and C57BL/6KitWv/+ mice. White-spotted (W or Wv) F1 mice were intercrossed to obtain Rag-2−/−γc−KitW/+ or Rag-2−/−γc−KitWv/+ mice. Mice were typed for Rag-2 deficiency by lack of T and B cells in the peripheral blood by flow cytometry and for γc deficiency by polymerase chain reaction (PCR) on genomic DNA as described.19 R−γ−KitW/Wv mice were obtained by crossing Rag-2−/−γc−KitW/+ to Rag-2−/−γc−KitWv/+ mice. KitW/W Tg(EPO) mice (strain, mixed WB and C57BL/6 background) were bred as described.17 The EPO transgene, its properties, and the promoter have been described previously.20
Flow cytometry and cell sorting
Cells were stained, analyzed, or sorted using Calibur analysis or Aria cell sorter instruments (BD Biosciences [BD], Heidelberg, Germany) as described.21,22 Briefly, cells were blocked with 500 μg/mL mouse IgG (Dianova, Hamburg, Germany), prior to specific antibody staining. Peritoneal exudate cells were harvested by peritoneal lavage with 10 mL warm (37°C) PBS/5% FCS and stained with FITC-labeled anti-T1 and PE-labeled anti-FcϵRI antibodies. Peripheral-blood leukocytes were enriched as described23 and analyzed for the presence of lymphocytes using biotinylated anti-CD45, PE-Cy7–labeled anti-CD3 (all BD Pharmingen, San Diego, CA) antibodies. Second-step reagent was streptavidin-APC (Molecular Probes, Carlsbad, CA). Splenic mast cells were analyzed by staining of spleen cell suspensions with a FITC-labeled lineage (lin) cocktail (anti-CD4, anti-CD8, anti-CD19, anti-Ter119), APC-labeled anti-Kit (all BD Pharmingen), and PE-labeled anti-FcϵRI (eBioscience, San Diego, CA). Mast cells were identified by their lin−Kit+FcϵRI+ phenotype. Numbers of lymphocytes in the blood and mast cells in the spleen were calculated by the addition of a defined number of flow cytometry beads (Calibrite; BD) to the stained cells. Lymphocytes were purified negatively by staining of spleen cells with a FITC-labeled myeloid-erythroid lineage cocktail (anti–Mac-1, anti–Gr-1, and anti-Ter119; all BD Pharmingen) followed by magnetic bead (Dynal Biotech, Oslo, Norway) depletion. Subsequently, cells were stained with APC-labeled anti-Kit, and Mac-1−Gr-1−Ter119−Kit−cells (ie, the nonmyeloid, nonerythroid, non–mast cell fraction) were cell-sorter purified. This fraction was composed of 53% B cells, 41% T cells, and the remaining cells being non-T and non-B cells (presumably natural killer [NK] cells). Kit+ cells were excluded from the sort to prevent transfer of contaminating wild-type mast cells together with the lymphocytes.
Adoptive cell transfers
Each mouse received 3 × 107 splenic lymphocytes by intravenous injections. Beginning on day 5 after lymphocyte transfer, mice were treated with PMA or acetone for 6 weeks. Numbers of lymphocytes were measured in the blood 13 days after transfer (not shown) and at the end of the experiment (6 weeks after transfer) in blood (Figure 6), spleen, and lymph nodes (not shown). Bone marrow mast cell cultures (BMMCs) were established as described.22 Because the relative contribution of local mast cell expansion versus increased colonization of mast cell progenitors from the circulation to the total mast cell increase in the inflamed skin is not fully understood, we choose intravenous cell transfer. Mice received a total of 3 intravenous BMMC injections (cell numbers were 1 × 107 on day 0, 2 × 106 on day 14, and 2 × 106 on day 22), and mast cell numbers in the skin (not shown) and the spleen (Figure 6) were determined on day 30.
Skin inflammation
One ear of each animal was treated 3 times per week on Monday, Wednesday, and Friday over a period of 6 weeks with 10 μg PMA (Sigma, Taufkirchen, Germany) dissolved in acetone (solvent). For controls, the contralateral ears were treated with solvent alone. Before each PMA treatment, ear thickness was determined using a caliper (Käfer Messuhrenfabrik, Villingen Schwenningen, Germany). After 6 weeks (Figures 4–5), mice were killed, and ear skin was processed for histology. The study protocol was approved by the Regierungsprasidium Tubingen (permit no. 761).
Histochemistry, immunofluorescence, and determination of mast-cell numbers
Histochemical analysis of tissue mast cells was done as described.22 Mast cells were also identified on acetone-fixed cryosections after blocking with 0.1 mg/mL mouse IgG in PBS/1% BSA using APC-labeled anti-Kit. The presence of granulocytes was determined by staining with FITC-labeled anti–Gr-1 (not shown). Tissue sections shown in Figures 2 and 4 were inspected with an Axioskop microscope using objectives with 10 × (0.30 aperture) and 20 × (0.50 aperture) magnification, respectively, at room temperature in air. Microscope and objectives were from Zeiss (Oberkochen, Germany). Fluorescence photomicrographs of berberine sulfate–stained cytospin preparations (Figure 2) were taken using the ORCA ER camera (Hamamatsu Photonics, Herrsching, Germany) and processed using Openlab software (Improvision, Coventry, England). Light microscopy photomicrographs of toluidin staining (Figure 4) were taken using the OM-11 color camera (Olympus, Hamburg, Germany).
RT-PCR
Ears and peritoneal exudate cells were homogenized in RNAzol (WAK-Chemie, Steinbach, Germany), and cDNA was prepared as described.12 Primers for reverse transcription (RT)–PCR were used as follows: Hprt sense, 5′-GCTGGTGAAAAGGACCTCT-3′; Hprt antisense, 5′-CACAGGACTAGAACACCTGC-3′; Mc-cpa sense, 5′-ACACAGGATCGAATGTGGAG-3′; Mc-cpa antisense, 5′-TAATGCAGGACTTCATGAGC-3′. Southern blot probe for the Mc-cpa PCR product was 5′-CCTCCTAACCACCAGGACCT-3′.
Results
Single and compound Kit mouse mutants
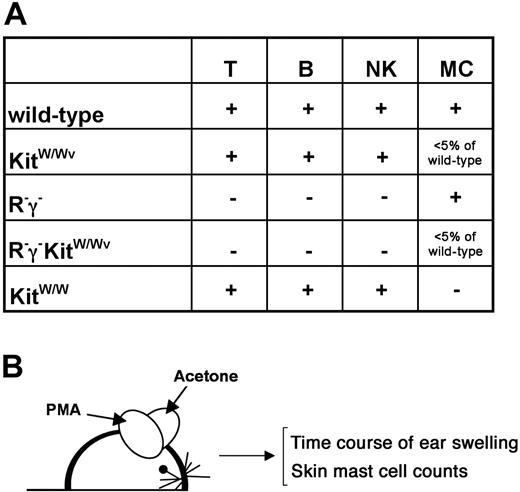
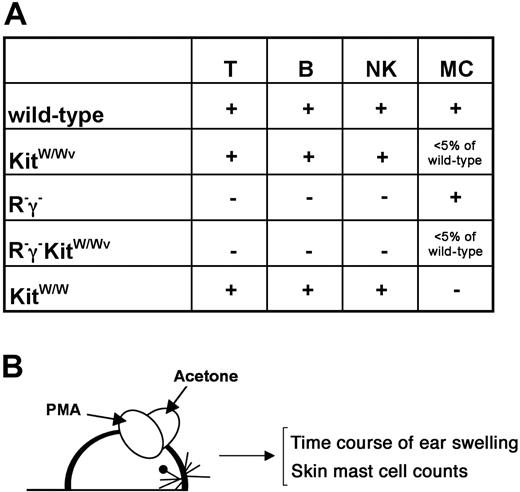
To investigate the role of Kit signaling strength in mast cells and the role of lymphocytes in the regulation of mast cell numbers in chronically inflamed skin, we generated a series of informative single and compound mutants (Figure 1A). Skin inflammation was induced in the following mouse strains: (1) mice wild type for Kit, Rag-2, and γc in which normal Kit signaling was permissive, and all lymphocytes subsets were present; (2) KitW/Wv mice in which Kit expression and signaling were impaired but not completely abrogated, and all lymphocytes subsets were present; (3) Kit wild-type mice lacking T, B, and NK cells (Rag-2−/−γc− [termed R−γ−]); (4) KitW/Wv mice lacking T, B, and NK cells (Rag-2−/−γc−KitW/Wv [termed R−γ−KitW/Wv]); and (5) KitW/W (ie, Kit cell surface null having peripheral T, B, and NK cells).
Mouse mutants and experimental outline to assess mast cells in normal and inflamed skin. (A) Genotypes and their impact on lymphocytes (T cells, B cells, NK cells) and on mast cells are indicated. The generation of new compound mutants is explained in the text. (B) Experimental outline. Mice were treated topically on one ear with PMA in acetone and on the contralateral ear with acetone (solvent) alone 3 times per week. Ear thickness was measured in regular intervals (see Figure 3) during the course of the treatment. After 6 weeks, numbers of mast cells in the ears were determined histologically.
Mouse mutants and experimental outline to assess mast cells in normal and inflamed skin. (A) Genotypes and their impact on lymphocytes (T cells, B cells, NK cells) and on mast cells are indicated. The generation of new compound mutants is explained in the text. (B) Experimental outline. Mice were treated topically on one ear with PMA in acetone and on the contralateral ear with acetone (solvent) alone 3 times per week. Ear thickness was measured in regular intervals (see Figure 3) during the course of the treatment. After 6 weeks, numbers of mast cells in the ears were determined histologically.
Mice of all genotypes where treated with PMA 3 times per week over a period of 6 weeks (Figure 1B). As a control, the contralateral ear of each mouse was treated with solvent alone. During the course of the treatment, ear thickness was determined as a measure of inflammation, and, after 4 to 6 weeks of treatment, mast cell numbers were counted by toluidine blue staining on skin sections of both ears.
Mast cell compartments in Kit and lymphocyte mutants
Mice bearing the viable KitW/Wv genotype have strongly reduced numbers (approximately 1% of wild-type mice) of mature dermal mast cells and lack peritoneal mast cells6,15 (Figure 2). In contrast to the widely used KitW/Wv mouse, up to now mast cells have not been analyzed in adult KitW/W mice. Cytospins of peritoneal exudate cells (PECs) were analyzed by toluidine blue reactivity which results in the mast cell–specific, metachromatic staining (Figure 2A-D) and by reactivity with the heparin-binding dye berberine sulfate24 (Figure 2E-H). To include cell numbers larger than those accessible on cytospins, PECs were also analyzed by flow cytometry using the mast cell marker T125 and the high-affinity IgE receptor (FcϵRI) (Figure 2I-L). Kit was excluded as a mast cell marker because it is only weakly or not at all expressed on hematopoietic cells in KitW/Wv and KitW/W mice, respectively.21
Peritoneal and skin mast cells in Kit mutant mice. Peritoneal exudate cells (PECs) (A-P) and skin (Q-T) from Kit+/+ (A,E,I,M,Q), KitW/Wv (B,F,J,N,R), R−γ−KitW/Wv (C,G,K,O,S), and KitW/W (D,H,L,P,T) mice were analyzed for the presence of mast cells. PEC cytospins were stained with toluidine blue (A-D) and berberine sulfate (E-H). Absence of mast cells in all Kit mutants is evident by lack of staining with these dyes. Scale bars in A and E correspond to 60 μm. (I-L) Flow cytometric analysis of PECs for expression of the mast cell markers T1 and FcϵRI. T1+FcϵRI+ cells were absent in all Kit mutants. Cells on the diagonal, present in all plots, are myeloid cells that are unaffected by mutations in Rag-2, γc, or Kit. Cells on the bottom of the dot plots are lymphocytes that are absent in R−γ−KitW/Wv owing to the mutations in Rag-2, and γc (M-T). Mice were 12 to 13 weeks of age. RT-PCR analysis for expression of mast cell carboxypeptidase A (Mc-cpa), a mast cell–specific gene, as a sensitive tool to detect mast cells in PECs (M-P) and ear skin (Q-T). The triangles indicate 5 cDNA dilutions in 1:10 steps. In line with the analysis shown in panels A through L, PECs lacked Mc-cpa expression in all Kit mutants (N-P). In contrast, Mc-cpa expression was detectable in KitW/Wv (R) and R−γ−KitW/Wv (S) mice. This expression was 10- to 100-fold reduced compared with Kit+/+ skin (Q). Mc-cpa expression was undetectable in the skin of KitW/W mice (T). Mice were 24 weeks (Kit+/+ [C57BL/6]), 12 weeks (KitW/Wv), 31 weeks (R−γ−KitW/Wv), and 17 weeks (KitW/W) old.
Peritoneal and skin mast cells in Kit mutant mice. Peritoneal exudate cells (PECs) (A-P) and skin (Q-T) from Kit+/+ (A,E,I,M,Q), KitW/Wv (B,F,J,N,R), R−γ−KitW/Wv (C,G,K,O,S), and KitW/W (D,H,L,P,T) mice were analyzed for the presence of mast cells. PEC cytospins were stained with toluidine blue (A-D) and berberine sulfate (E-H). Absence of mast cells in all Kit mutants is evident by lack of staining with these dyes. Scale bars in A and E correspond to 60 μm. (I-L) Flow cytometric analysis of PECs for expression of the mast cell markers T1 and FcϵRI. T1+FcϵRI+ cells were absent in all Kit mutants. Cells on the diagonal, present in all plots, are myeloid cells that are unaffected by mutations in Rag-2, γc, or Kit. Cells on the bottom of the dot plots are lymphocytes that are absent in R−γ−KitW/Wv owing to the mutations in Rag-2, and γc (M-T). Mice were 12 to 13 weeks of age. RT-PCR analysis for expression of mast cell carboxypeptidase A (Mc-cpa), a mast cell–specific gene, as a sensitive tool to detect mast cells in PECs (M-P) and ear skin (Q-T). The triangles indicate 5 cDNA dilutions in 1:10 steps. In line with the analysis shown in panels A through L, PECs lacked Mc-cpa expression in all Kit mutants (N-P). In contrast, Mc-cpa expression was detectable in KitW/Wv (R) and R−γ−KitW/Wv (S) mice. This expression was 10- to 100-fold reduced compared with Kit+/+ skin (Q). Mc-cpa expression was undetectable in the skin of KitW/W mice (T). Mice were 24 weeks (Kit+/+ [C57BL/6]), 12 weeks (KitW/Wv), 31 weeks (R−γ−KitW/Wv), and 17 weeks (KitW/W) old.
On the basis of toluidine blue and berberine staining, and on flow cytometry, peritoneal mast cells were detectable in Kit+/+ (Figure 2A,E,I) and in R−γ− mice (not shown). In contrast, peritoneal mast cells were undetectable in all Kit mutants, that is, KitW/Wv (Figure 2B,F,J), R−γ−KitW/Wv (Figure 2C,G,K), and KitW/W (Figure 2D,H,L). Hence, the absence of lymphocytes did not affect the peritoneal mast cell compartment. In addition, the data confirmed the massive reduction of mast cells in KitW/Wv mice. At this level of analysis, there was no difference comparing hypomorphic KitW/Wv and null KitW/W mice with regard to mast cell development.
Gene expression analysis for mast cell–specific genes is a sensitive way to detect mast cells.26 The mast cell carboxypeptidase A (Mc-cpa) is strongly and specifically expressed in the mast cell lineage27 (references in Feyerabend et al22 ). Therefore, Mc-cpa expression was analyzed by RT-PCR in PECs (Figure 2M-P) or ears (Figure 2Q-T). In wild-type mice (Figure 2M,Q), Mc-cpa mRNA was strongly expressed in PECs and in skin. Consistent with the absence of mast cells in the peritoneal cavity of KitW/Wv mice,6 Mc-cpa mRNA was undetectable in KitW/Wv (Figure 2N) and R−γ−KitW/Wv (Figure 2O) mice. Not surprisingly, KitW/W PECs also lacked Mc-cpa mRNA (Figure 2P), indicating a complete absence of peritoneal mast cells in KitW/W mice.
In the ears, Mc-cpa was again strongly expressed in wild-type mice (Figure 2Q). Confirming previous results,26 we detected Mc-cpa mRNA in the skin of both KitW/Wv mutants (Figure 2R,S). Titration of cDNA templates suggested that Mc-cpa expression was 10- to 100-fold lower in the ears of KitW/Wv mutants compared with wild-type mice (Figure 2R,S). Interestingly, in the ears of KitW/W mice Mc-cpa expression was undetectable by RT-PCR (Figure 2T). These data suggest that the residual Kit function in KitW/Wv mice is required for the low but significant mast cell production under steady state conditions in the KitW/Wv mutant.
Mast-cell dependency of initial versus long-term ear swelling after PMA treatment
PMA treatment elicits acute and chronic inflammatory responses, akin to irritative dermatitis, by nonspecific activation of a variety of PMA receptors (reviewed in Boutwell28 and Brose and Rosenmund29 ). The resulting immunologically nonspecific inflammation, measured by immediate (< 36 hours) tissue swelling, is amplified by mast cells after PMA treatment.30 To measure the impact of other immune cells on this inflammation process, we determined time course and extent of ear thickness in all mutants after PMA treatment (Figure 3).
Ear swelling during PMA treatment. Ear thickness of PMA-treated ears (■) and solvent-treated contralateral ears (▴) was monitored with a caliper over a period of 6 weeks. Means and SDs of 3 mice per genotype are shown. Data are representative for 1 of 2 complete and independent experiments. Mice used were 13 weeks (Kit+/+ [C57BL/6]), 8 weeks (KitW/Wv), 10 weeks (R−γ−), 16 weeks (R−γ−KitW/Wv), 2 × 9 weeks, and 1 × 16 weeks (KitW/W) old.
Ear swelling during PMA treatment. Ear thickness of PMA-treated ears (■) and solvent-treated contralateral ears (▴) was monitored with a caliper over a period of 6 weeks. Means and SDs of 3 mice per genotype are shown. Data are representative for 1 of 2 complete and independent experiments. Mice used were 13 weeks (Kit+/+ [C57BL/6]), 8 weeks (KitW/Wv), 10 weeks (R−γ−), 16 weeks (R−γ−KitW/Wv), 2 × 9 weeks, and 1 × 16 weeks (KitW/W) old.
Ear swelling of fully immune-competent mice peaked at day 10 and declined thereafter to approximately double the normal thickness compared with nontreated contralateral ears (Figure 3A). Such strong initial reactions were not observed in any of the immune-compromised mice (Figure 3B-E), suggesting that mast cells and lymphocytes can both contribute to the initial swelling in the first 10 days. Beginning on day 12, and for the remainder of the observation time, ear thickness of wild-type (Figure 3A), KitW/Wv (Figure 3B), R−γ− (Figure 3C), and KitW/W (Figure 3E) mice had at least doubled compared with the starting point (swelling ≥ 0.5 mm). In contrast, PMA-treated ears of R−γ−KitW/Wv mice showed reduced swelling (∼ 0.35 mm) in the long term (Figure 3D). Collectively, initial ear swelling was augmented by the presence of immune cells (either T, B, NK, and/or mast cells) (compare Figure 3A with B-E). Long-term ear swelling occurred in all mutants, but in the absence of all adaptive (T and B cells) and parts of innate (NK and mast cells) immune cells long-term ear swelling was reduced.
To obtain information about other cell types that are recruited during this inflammatory response, we examined infiltration of Gr-1+ leukocytes to the sites of chronic dermatitis. Immunofluorescence analysis of skin sections revealed an accumulation of polymorphonuclear Gr-1+ granulocytes in all treated ears, indicating that granulocyte recruitment was mast cell and lymphocyte independent (not shown).
Kit-mediated signals are pivotal for mast-cell accumulation during chronic dermatitis
To assess the contribution of Kit-mediated signals to the mast cell accumulation during chronic dermatitis, ears of PMA-treated mice of all genotypes were analyzed histochemically for the presence of mast cells (Figures 4–5). Skin thickness of PMA-treated ears (Figure 4B,D,F,H) was clearly increased compared with solvent-treated control ears (Figure 4A,C,E,G) visualizing the findings obtained by micrometer measurements (Figure 3). Mast cells were identified by their morphology and by their metachromatic staining with toluidine blue. Mast cells in control ears were only present in wild-type (Figure 4A) and R−γ− (not shown) mice. Following control solvent treatment, we could not detect skin mast cells in KitW/Wv (Figure 4C), R−γ−KitW/Wv (Figure 4E), or KitW/W (Figure 4G) mice, demonstrating the paucity or absence of mast cells in the healthy skin of these mutants. As expected, PMA treatment resulted in increased mast cell numbers in wild-type mice (Figure 4B). Mast cell numbers increased not only in wild-type mice but also in KitW/Wv (Figure 4D) and R−γ−KitW/Wv (Figure 4F) mice. Interestingly, mast cells were undetectable in skin sections of KitW/W mice (Figure 4H).
Mast-cell accumulation and skin morphology after local PMA treatment. Six weeks after treatment of ears with solvent (acetone) (A,C,E,G) or with PMA (B,D,F,H) ear sections were stained with toluidine blue to assess the overall skin morphology and to obtain mast cell counts. Strongly increased skin thickness is apparent in all PMA-treated ears (B-H). Arrows indicate metachromatically stained mast cells in PMA-treated ears. Mast cells accumulated exclusively in the ears of Kit+/+ (B) and KitW/Wv (D) and R−γ−KitW/Wv (F) mice. Mice used were 8 weeks (Kit+/+ [WB]), 11 weeks (KitW/Wv), 25 weeks (R−γ−KitW/Wv), and 13 weeks (KitW/W) of age. The skin of KitW/W mice that lack all Kit cell-surface expression was completely devoid of mast cells. This was true before (G) and after (H) PMA-induced inflammation. The scale bar (A) applies to all panels and corresponds to 40 μm.
Mast-cell accumulation and skin morphology after local PMA treatment. Six weeks after treatment of ears with solvent (acetone) (A,C,E,G) or with PMA (B,D,F,H) ear sections were stained with toluidine blue to assess the overall skin morphology and to obtain mast cell counts. Strongly increased skin thickness is apparent in all PMA-treated ears (B-H). Arrows indicate metachromatically stained mast cells in PMA-treated ears. Mast cells accumulated exclusively in the ears of Kit+/+ (B) and KitW/Wv (D) and R−γ−KitW/Wv (F) mice. Mice used were 8 weeks (Kit+/+ [WB]), 11 weeks (KitW/Wv), 25 weeks (R−γ−KitW/Wv), and 13 weeks (KitW/W) of age. The skin of KitW/W mice that lack all Kit cell-surface expression was completely devoid of mast cells. This was true before (G) and after (H) PMA-induced inflammation. The scale bar (A) applies to all panels and corresponds to 40 μm.
Steady state and inflammation-induced mast cell numbers in the skin of Kit+/+, KitW/Wv, R−γ−, R−γ−KitW/Wv, and KitW/W mice. After 6 weeks of PMA-induced chronic dermatitis, numbers of metachromatic mast cells were determined on toluidine blue–stained sections of ear skin. Closed symbols represent control (acetone)–treated ears, and open symbols represent PMA-treated ears. The figure summarizes data from 2 independent experiments with 3 mice per genotype and experiment (total number of mice = 6). Horizontal lines indicate the mean cell number for each group of mice (A-E). Numbers in healthy skin and the inflammation-induced increase were comparable in Kit+/+ (A) and R−γ− (C) mice. Numbers in healthy skin were very low in untreated KitW/Wv (B) and R−γ−KitW/Wv (D) skin. In PMA-treated skin, the increase in mast cell numbers was approximately 10-fold larger in KitW/Wv compared with R−γ−KitW/Wv mice. In the skin from KitW/W mice, mast cells were undetectable and remained absent after PMA-induced chronic dermatitis (E). The differences in mast cell counts comparing untreated (closed symbols) and PMA-treated (open symbols) skin samples had significant one-sided P values (< .05) (Mann-Whitney test) in panels A through C. The P value (.09) in panel D was not significant. Data from mice of 2 independent experiments were pooled in this figure. Mice used in these experiments were 13 to 21 weeks (Kit+/+ [3 × C57BL/6; 3 × WB]), 8 to 19 weeks (KitW/Wv), 10 to 19 weeks (R−γ−), 9 to 16 weeks (R−γ−KitW/Wv), or 9 to 36 weeks (KitW/W) of age.
Steady state and inflammation-induced mast cell numbers in the skin of Kit+/+, KitW/Wv, R−γ−, R−γ−KitW/Wv, and KitW/W mice. After 6 weeks of PMA-induced chronic dermatitis, numbers of metachromatic mast cells were determined on toluidine blue–stained sections of ear skin. Closed symbols represent control (acetone)–treated ears, and open symbols represent PMA-treated ears. The figure summarizes data from 2 independent experiments with 3 mice per genotype and experiment (total number of mice = 6). Horizontal lines indicate the mean cell number for each group of mice (A-E). Numbers in healthy skin and the inflammation-induced increase were comparable in Kit+/+ (A) and R−γ− (C) mice. Numbers in healthy skin were very low in untreated KitW/Wv (B) and R−γ−KitW/Wv (D) skin. In PMA-treated skin, the increase in mast cell numbers was approximately 10-fold larger in KitW/Wv compared with R−γ−KitW/Wv mice. In the skin from KitW/W mice, mast cells were undetectable and remained absent after PMA-induced chronic dermatitis (E). The differences in mast cell counts comparing untreated (closed symbols) and PMA-treated (open symbols) skin samples had significant one-sided P values (< .05) (Mann-Whitney test) in panels A through C. The P value (.09) in panel D was not significant. Data from mice of 2 independent experiments were pooled in this figure. Mice used in these experiments were 13 to 21 weeks (Kit+/+ [3 × C57BL/6; 3 × WB]), 8 to 19 weeks (KitW/Wv), 10 to 19 weeks (R−γ−), 9 to 16 weeks (R−γ−KitW/Wv), or 9 to 36 weeks (KitW/W) of age.
Next, we obtained mast cell counts that were normalized on cartilage length (Figure 5). In wild-type mice (Figure 5A) and in R−γ− mice (Figure 5C), 6 of 6 mice analyzed for each genotype showed a 3- to 4-fold increase in mast cells after PMA treatment (Kit+/+ mice control ears [mean ± SD, 540 ± 274 mast cells/cm; Kit+/+ mice PMA-treated ears, 1733 ± 454 mast cells/cm; R−γ− mice control ears, 372 ± 96 mast cells/cm; R−γ− mice PMA-treated ears, 999 ± 373 mast cells/cm). Mast cells also accumulated in 6 of 6 PMA-treated ears from KitW/Wv mice (Figure 5B) (KitW/Wv mice control ears, 1.3 ± 0.8 mast cells/cm; KitW/Wv mice PMA-treated ears, 179 ± 136 mast cells/cm) as reported previously.15 Although absolute mast cell numbers remained lower in the skin of KitW/Wv mice compared with wild-type or R−γ− mice, the relative increase was larger in KitW/Wv compared with wild-type or R−γ− mice. Interestingly, PMA treatment did not lead to the appearance of mast cells in the skin of KitW/W mice (Figure 5E) (KitW/W mice control ears, 0 mast cell/cm; KitW/W mice PMA-treated ears, 0 mast cell/cm), demonstrating an essential role for Kit-mediated signals in mast cell development in healthy and inflamed skin.
Role of lymphocytes and the common γ chain for mast cells in KitW/Wv mice
Analysis of R−γ− mice (Figure 5C) indicated that the absence of lymphocytes and lack of γc expression did not impair the number of constitutive skin mast cells or their increase following skin inflammation in mice expressing wild-type levels of Kit. Thus, in contrast to peritoneal mast cells,31 γc expression is not required for normal numbers of skin mast cells. Because Kit signaling can act in marked synergy with γc signaling in other cell types such as thymocytes,32 we next examined the inflammation-driven mast cell increase in combined Rag-2 and γc mutants on a KitW/Wv background (R−γ−KitW/Wv mice). In these mutants, the overall increase in mast cell numbers was strongly blunted (Figure 5D) (control ears, 2 ± 2 mast cells/cm, PMA, 12 ± 19 mast cells/cm) compared with KitW/Wv mice (Figure 5B). Moreover, 2 of 6 R−γ−KitW/Wv mice did not respond at all. Thus, on a KitW/Wv background, the presence of lymphocytes or γc expression is important for the regulation of mast cell numbers in inflamed skin.
To distinguish between effects mediated by lack of Rag-2 (ie, loss of T and B cells) or γc expression (ie, loss of signaling via γc-dependent cytokines), we performed 2 sets of further experiments. In the first set, cell-sorter purified, wild-type (R+γ+Kit+/+) lymphocytes (see “Materials and methods”) were injected intravenously into R−γ−KitW/Wv mice. This adoptive transfer lead to reconstitution of circulating T cells in R−γ−KitW/Wv mice reaching about 50% (2157 ± 819 T cells/μL blood) (Figure 6D) of the numbers found in Kit+/+ (3960 ± 1017 T cells/μL blood) (Figure 6A) or KitW/Wv (4250 ± 780 T cells/μL blood) (Figure 6B) mice. No lymphocytes were detectable in R−γ−KitW/Wv mice (Figure 6C). Ears in these groups of mice were subjected to PMA treatment over a period of 6 weeks. In agreement with the experiments shown in Figure 5, Kit+/+ showed a robust increase in skin mast cell numbers (control ears, 318 ± 64 mast cells/cm; PMA-treated ears, 1526 ± 139 mast cells/cm) (Figure 6E). Likewise, KitW/Wv demonstrated a marked response (control ears, 1.7 ± 2.8 mast cells/cm; PMA-treated ears, 216 ± 80 mast cells/cm) (Figure 6F), whereas R−γ−KitW/Wv mice showed only a very modest mast cell response (control ears, 1 ± 1.7 mast cells/cm; PMA-treated ears, 22 ± 6 mast cells/cm) (Figure 6G). Lymphocyte-reconstituted R−γ−KitW/Wv mice harbored 4 ± 4.5 mast cells/cm in control ears and 95 ± 71 mast cells/cm in PMA-treated ears (Figure 6H). Although the comparison of the PMA-induced cell numbers in non–lymphocyte-reconstituted R−γ−KitW/Wv (open symbols in Figure 6G) versus in lymphocyte-reconstituted R−γ−KitW/Wv mice (open symbols in Figure 6H) has a P value of only .2 (Mann Whitney rank test) and hence does not reach statistical significance, it should be noted that 2 of 3 mice showed mast cell numbers above 100 cells/cm (106 and 160). We did not observe similarly high mast cell numbers in any of the nonreconstituted, PMA-treated R−γ−KitW/Wv ears (Figures 5–6), suggesting that the addition of lymphocytes to R−γ−KitW/Wv mice might promote mast cell accumulation in the inflamed skin.
Role of lymphocytes and the common γ chain for mast cells in KitW/Wv mice. Adoptive transfer of wild-type (R+γ+Kit+/+) splenic lymphocytes into R−γ−KitW/Wv mice (D) lead to approximately 50% reconstitution of circulating T cells compared with Kit+/+ (A) or KitW/Wv (B) mice. Nonreconstituted R−γ−KitW/Wv mice are shown as controls (C). Ears in these groups of mice were subjected to PMA treatment over a period of 6 weeks, and mast cell numbers in treated and untreated ears from all mice were determined by histology (E-H). The differences in mast cell counts comparing untreated (closed symbols) and PMA-treated (open symbols) skin samples had significant one-sided P values (P = .05, Mann-Whitney test) in panels E through H. The P value (.2) comparing the PMA-treated skins in panel G versus panel H was not significant (see text). Kit+/+ were 13-week-old C57BL/6 mice (A,E). KitW/Wv (B,F), noninjected R−γ−KitW/Wv (C,G), and lymphocyte-recipient R−γ−KitW/Wv (D,H) mice were 17 to 20 weeks old. Donors (D,H) were 25-week-old C57BL/6 mice. To determine the possible role of γc for the defective mast cell reconstitution in R−γ−KitW/Wv mice, wild-type (R+γ+Kit+/+) BMMCs were injected repeatedly (see “Material and methods” for cell numbers, frequency of injections, and duration of the experiment) into R−γ−KitW/Wv (J) and KitW/Wv (K) mice. Numbers of mast cells in the spleen, a site that is more effectively colonized than skin following intravenous transfer of BMMCs,33 were determined by flow cytometry in noninjected controls (solid symbols in I-K) and in injected R−γ−KitW/Wv (J) and KitW/Wv (K). Age of the mice was 17 weeks (C57BL/6) (I), 9 to 11 weeks (noninjected R−γ−KitW/Wv) (J), 13 to 30 weeks (BMMC-injected R−γ−KitW/Wv) (J), 21 weeks (noninjected KitW/Wv) (K), and 39 weeks (BMMC-injected KitW/Wv) (K). Mast cells appeared in the spleens of both types of recipients, but their numbers were at least one order of magnitude higher in KitW/Wv compared with R−γ−KitW/Wv. The one-sided P value (.004) of the comparison of the BMMC-injected animals in panel J versus panel K by Mann-Whitney test was significant. Horizontal lines indicate the mean cell number for each group of mice (A-K). Hence, γc-competent mast cells did not rescue the defective mast cell reconstitution in R−γ−KitW/Wv mice.
Role of lymphocytes and the common γ chain for mast cells in KitW/Wv mice. Adoptive transfer of wild-type (R+γ+Kit+/+) splenic lymphocytes into R−γ−KitW/Wv mice (D) lead to approximately 50% reconstitution of circulating T cells compared with Kit+/+ (A) or KitW/Wv (B) mice. Nonreconstituted R−γ−KitW/Wv mice are shown as controls (C). Ears in these groups of mice were subjected to PMA treatment over a period of 6 weeks, and mast cell numbers in treated and untreated ears from all mice were determined by histology (E-H). The differences in mast cell counts comparing untreated (closed symbols) and PMA-treated (open symbols) skin samples had significant one-sided P values (P = .05, Mann-Whitney test) in panels E through H. The P value (.2) comparing the PMA-treated skins in panel G versus panel H was not significant (see text). Kit+/+ were 13-week-old C57BL/6 mice (A,E). KitW/Wv (B,F), noninjected R−γ−KitW/Wv (C,G), and lymphocyte-recipient R−γ−KitW/Wv (D,H) mice were 17 to 20 weeks old. Donors (D,H) were 25-week-old C57BL/6 mice. To determine the possible role of γc for the defective mast cell reconstitution in R−γ−KitW/Wv mice, wild-type (R+γ+Kit+/+) BMMCs were injected repeatedly (see “Material and methods” for cell numbers, frequency of injections, and duration of the experiment) into R−γ−KitW/Wv (J) and KitW/Wv (K) mice. Numbers of mast cells in the spleen, a site that is more effectively colonized than skin following intravenous transfer of BMMCs,33 were determined by flow cytometry in noninjected controls (solid symbols in I-K) and in injected R−γ−KitW/Wv (J) and KitW/Wv (K). Age of the mice was 17 weeks (C57BL/6) (I), 9 to 11 weeks (noninjected R−γ−KitW/Wv) (J), 13 to 30 weeks (BMMC-injected R−γ−KitW/Wv) (J), 21 weeks (noninjected KitW/Wv) (K), and 39 weeks (BMMC-injected KitW/Wv) (K). Mast cells appeared in the spleens of both types of recipients, but their numbers were at least one order of magnitude higher in KitW/Wv compared with R−γ−KitW/Wv. The one-sided P value (.004) of the comparison of the BMMC-injected animals in panel J versus panel K by Mann-Whitney test was significant. Horizontal lines indicate the mean cell number for each group of mice (A-K). Hence, γc-competent mast cells did not rescue the defective mast cell reconstitution in R−γ−KitW/Wv mice.
In the second set of experiments, we addressed the possibility that the poor mast cell increase in R−γ−KitW/Wv mice was due to lack of γc expression in mast cells31 in these mutants. To this end, we transplanted γc-competent (R+γ+Kit+/+) BMMCs into R−γ−KitW/Wv and KitW/Wv hosts. Despite repeated intravenous injections of BMMCs (see “Material and methods” for reasoning for route of administration, cell numbers, and frequency of injections), we failed to detect a significant increase in mast cell numbers in the ears of both types of recipients (R−γ−KitW/Wv without BMMCs [n = 3], 1.3 ± 1.5 mast cells/cm; R−γ−KitW/Wv with BMMCs [n = 5], 3.2 ± 5.5 mast cells/cm; KitW/Wv without BMMCs [n = 3], 7.7 ± 6.5 mast cells/cm; KitW/Wv with BMMCs [n = 5], 5.4 ± 3.4 mast cells/cm). Moreover, R−γ−KitW/Wv mice transplanted with wild-type (R+γ+Kit+/+) BMMCs and challenged with PMA (as described for Figure 6E-H) also failed to generated skin mast cells (not shown).
These findings are in agreement with previous experiments that revealed very inefficient mast cell reconstitution in the skin following intravenous transfer.33 However, the spleen has been reported to be a more effective site of mast cell reconstitution following intravenous transfer of BMMCs.33 We therefore compared by flow cytometry mast cell numbers in the spleens of the same R−γ−KitW/Wv and KitW/Wv recipient mice that had received γc-competent (R+γ+Kit+/+) BMMCs. Mast cells were present in the spleens of both types of recipients, but, interestingly, numbers of mast cells were at least one order of magnitude higher in KitW/Wv (1648 ± 780 mast cells/cm) compared with R−γ−KitW/Wv (87 ± 112 mast cells/cm) recipients (Figure 6J,K) (P = .004). Because γc-competent BMMCs reconstituted KitW/Wv mice well, but R−γ−KitW/Wv only poorly, these experiments support the notion that lymphocytes contribute to the regulation of mast cell compartments upon reconstitution of mast cell–deficient mice. Under these conditions, γc-competent mast cells did not rescue the defective mast cell reconstitution in R−γ−KitW/Wv mice.
Discussion
Despite the important contributions of mast cells to the pathophysiology of skin inflammation, little is known about the cellular and molecular signals that regulate mast cell numbers in inflamed skin. The crucial role of Kit and its ligand, Scf, for normal mast cell development is evident in mice bearing hypomorphic mutations in the genes encoding Kit6 or Scf.34 The mast cell paucity is, however, not absolute in the KitW/Wv mutant as shown by a significant enrichment of mast cells at sites of idiopathic14 or chemically15 induced chronic dermatitis. These findings raised the question whether residual Kit signaling, mediated by the KitW/Wv receptor, was facilitating this response or whether mechanisms unrelated to Kit signaling were able to drive mast cell accumulation under these conditions. Whatever the nature of these signals, they are insufficient to promote significant numbers of skin mast cells in KitW/Wv mice under nonpathologic conditions.
Here, we have taken advantage of the recently developed viable KitW/W mutants17 to address the role of Kit in the regulation of mast cell numbers during chronic dermatitis. Adult KitW/W mice have previously given insight into the crucial role of Kit in adult lymphopoiesis.12,35 Although in vitro–derived mast cell lines were used to characterize the loss of Kit expression in viable KitW/W mutants,12 mast cell compartments have not been analyzed in these mice. Because the hypomorphic KitW/Wv mutant has been widely used as a “mast cell–deficiency model,” we have now compared mast cells in KitW/W and KitW/Wv mice. The results show that KitW/W mice differ in several aspects from KitW/Wv mice. On the basis of mast cell protease expression (Figure 2) and histologic analysis (Figure 4), we confirm previous reports (reviewed in Tsai et al26 ) that KitW/Wv mice have very low but measurable numbers of skin but not peritoneal mast cells. In contrast, using the same criteria, we now find no evidence for skin mast cells in KitW/W mice. The absence of mast cells in the peritoneal cavity in both strains, but the presence of mast cells in the skin of KitW/Wv mice, suggests that skin mast cells are slightly less dependent on Kit signaling compared with peritoneal mast cells. However, the most dramatic difference between mast cells in KitW/Wv and KitW/W mice became only apparent following experimentally induced chronic dermatitis. Although mast cell numbers increased strongly in KitW/Wv mice, mast cells remained undetectable in KitW/W mice. These experiments demonstrate an active role of the KitWv protein in the regulation of mast cell numbers in response to skin inflammation. Collectively, Kit-mediated signals are essential for skin mast cell formation under steady state conditions and under inflammatory conditions.
Short- and long-term reactions during PMA-induced chronic dermatitis include ear swelling. PMA nonspecifically activates a wide variety of cell types28 by directly interacting with the signaling component protein kinase C and other PMA receptors.29 To determine whether the residual Kit function in KitW/Wv mast cells was in itself sufficient for the mast cell response to inflammation, we eliminated T, B, and NK cells from KitW/Wv mice by generating R−γ−KitW/Wv mice. In short-term reactions (≤ 30 hours) mast cells play a role in ear swelling.30 After 3 days of PMA treatment we observed a slower onset of ear swelling in all analyzed mutants. Thus, not only mast cells, but also T, B, or NK cells, are required for initial ear swelling. In contrast, after 15 days, only mice lacking T, B, and NK cells showed reduced swelling. Ear thickness was indistinguishable after 6 weeks of PMA treatment comparing Kit+/+ and KitW/Wv mice15 (Figure 3). The fact that R−γ− mice showed a similar swelling demonstrates that lymphocytes are not required for this skin reaction. Moreover, R−γ−KitW/Wv that had initially very few mast cells and constantly lacked lymphocytes showed the same degree of swelling.
The unexpected mast cell reconstitution defect in R−γ−KitW/Wv, when compared with KitW/Wv mice, might be due to lack of γc expression on mast cells or due to the absence of lymphocytes. Mice deficient for γc or a downstream signaling molecule Jak-3 were reported to have 2-fold reduced numbers of peritoneal mast cells.31 We did not notice a reduction in skin mast cells in R−γ− mice, thus ruling out an important function for γc in this mast cell population. However, γc signaling in mast cells might be important if the Kit signal is limiting. Although we cannot definitively exclude a role for γc under such conditions, the relative inability of adoptively transferred γc+ BMMCs to reconstitute splenic mast cells in R−γ−KitW/Wv mice (Figure 6) argues against a major role of γc in mast cell reconstitution. The inability of robust reconstitution of skin mast cells following intravenous transfer of BMMCs prevented a more direct test whether the same rules apply to the skin as shown here for the spleen.
Lack of lymphocytes played no role for the accumulation of mast cells in Kit+/+ mice but T, B, or NK cells were required on the KitW/Wv background. These data suggest that mast cells can expand in the skin by 2 mechanisms. The first possibility is a strong signal via Kit alone that can be transmitted by the wild-type (Kit+) but not by the kinase-weak (KitWv) receptor. The second route would require 2 signals, a weak Kit signal that could be transmitted via KitWv in KitW/Wv mice and a second signal that originates directly or indirectly from lymphocytes. It is conceivable that a weak Kit signal could also be generated in normal mice (Kit+/+) under conditions of limiting availability of Kit ligand. The strongly blunted mast cell response in R−γ−KitW/Wv compared with KitW/Wv mice agrees with a supportive role of lymphocytes in mast cell recruitment, generation, or local proliferation. In addition to T and B cells, NK cells that are absent in R−γ−KitW/Wv mice could also play a role. The lymphocyte support for mast cell expansion could occur via direct cellular interactions or lymphocyte-derived factors may drive mast cell expansion. In vitro, IL-3 allows outgrowth of mast cells from bone marrow or peripheral blood of KitW/Wv36 and KitW/W12 mice. Furthermore, perfusion of KitW/Wv mice with IL-3 normalizes skin mast cell numbers,37 and Kit ligand and IL-3 cooperate in the mast cell–driven worm expulsion.38 In KitW/Wv mice, T-cell–derived IL-3 might, therefore, be a crucial factor for mast cell accumulation during the course of chronic dermatitis.
Presently, it is difficult to distinguish whether the defect in KitW/W mice is due to reduced availability of circulating mast cell progenitors or due to impaired skin colonization of mast cell progenitors or due to impaired survival or abrogated proliferation of mast cells within the skin. After 6 weeks of PMA treatment of normal mice, histologic analysis revealed that skin mast cells lacked expression of a proliferation marker, phospho-histone H3 (Ser10) (data not shown), indicating that, at least at this time point, mast cells did not proliferate locally. It is, however, possible that mast cells proliferated locally at earlier time points during the course of the inflammation. Progenitors with in vitro mast cell potential are present in peripheral blood of adult KitW/W mice,12 but these cells have not been accessible for direct analysis, partly due to lack of Kit expression that is an important marker of stem/progenitor cells. Hence, the precise stage at which mast cell development is absolutely blocked in adult KitW/W mice has to await the identification or development of specific mast cell progenitor markers. These could also be useful to study mast cell progenitor mobilization and their recruitment to healthy and inflamed tissues.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Michael Huber, Freiburg, for generous supply of BMMC, Thorsten Feyerabend for proficient cell transfers, Dr Hans Jörg Fehling for critical reading of the manuscript, and members of the animal facility for careful mouse keeping.
This work was supported by the Landesstiftung (P-LS-AL/11) (C.W. and H.-R.R.) and the Landesgraduierten-Förderung (S.M.S.) Baden-Württemberg and by the Deutsche Forschungsgemeinschaft (DFG-RO754/2-2) (H.-R.R.).
Authorship
Contribution: C.W. and H.-R.R. designed research, analyzed data, and wrote the paper; C.W., S.B., S.M.S., and C.C. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hans-Reimer Rodewald, Institute for Immunology, University of Ulm, D-89081 Ulm, Germany; e-mail: hans-reimer.rodewald@uni-ulm.de.

![Figure 2. Peritoneal and skin mast cells in Kit mutant mice. Peritoneal exudate cells (PECs) (A-P) and skin (Q-T) from Kit+/+ (A,E,I,M,Q), KitW/Wv (B,F,J,N,R), R−γ−KitW/Wv (C,G,K,O,S), and KitW/W (D,H,L,P,T) mice were analyzed for the presence of mast cells. PEC cytospins were stained with toluidine blue (A-D) and berberine sulfate (E-H). Absence of mast cells in all Kit mutants is evident by lack of staining with these dyes. Scale bars in A and E correspond to 60 μm. (I-L) Flow cytometric analysis of PECs for expression of the mast cell markers T1 and FcϵRI. T1+FcϵRI+ cells were absent in all Kit mutants. Cells on the diagonal, present in all plots, are myeloid cells that are unaffected by mutations in Rag-2, γc, or Kit. Cells on the bottom of the dot plots are lymphocytes that are absent in R−γ−KitW/Wv owing to the mutations in Rag-2, and γc (M-T). Mice were 12 to 13 weeks of age. RT-PCR analysis for expression of mast cell carboxypeptidase A (Mc-cpa), a mast cell–specific gene, as a sensitive tool to detect mast cells in PECs (M-P) and ear skin (Q-T). The triangles indicate 5 cDNA dilutions in 1:10 steps. In line with the analysis shown in panels A through L, PECs lacked Mc-cpa expression in all Kit mutants (N-P). In contrast, Mc-cpa expression was detectable in KitW/Wv (R) and R−γ−KitW/Wv (S) mice. This expression was 10- to 100-fold reduced compared with Kit+/+ skin (Q). Mc-cpa expression was undetectable in the skin of KitW/W mice (T). Mice were 24 weeks (Kit+/+ [C57BL/6]), 12 weeks (KitW/Wv), 31 weeks (R−γ−KitW/Wv), and 17 weeks (KitW/W) old.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-08-039131/4/m_zh80120701220002.jpeg?Expires=1765150083&Signature=h4zezgRieiInQtnm1YZm4-Xl5gPcD5gMSQ09xtQig8jklQGehu7IFFDJbY~c7b8hB1ftCyv4udqr9ziHaKn~FNTV176vLfvjOaoBDNUh4fZIMNy2IA-k-7pORVx12Z7BubkImsw260zaYyCL1tK9-yaJywpB4RVyV3nD0DSmjWKak7EYz4ZBbogyjfpRKrdoQeEZsuKr6zPExgv7yTfgrWB~rOi6dOkaSElx1KZcs58Yqy8Mi1AT03si3jzExsWAQFTBlSMQqvkIWBsjjEKCWuKuS3Lsjvu3JlTRL1yyj9hax8cumuu-g1C0YAK-ILBRZjal9KkoEDE3c3ebs5hqoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Ear swelling during PMA treatment. Ear thickness of PMA-treated ears (■) and solvent-treated contralateral ears (▴) was monitored with a caliper over a period of 6 weeks. Means and SDs of 3 mice per genotype are shown. Data are representative for 1 of 2 complete and independent experiments. Mice used were 13 weeks (Kit+/+ [C57BL/6]), 8 weeks (KitW/Wv), 10 weeks (R−γ−), 16 weeks (R−γ−KitW/Wv), 2 × 9 weeks, and 1 × 16 weeks (KitW/W) old.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-08-039131/4/m_zh80120701220003.jpeg?Expires=1765150083&Signature=OvFnO7rE6eK7s3ynRgRJWZYgkUagP890XtKPwDJjtTeJYs8iYtbMucr8kd7VjBvA8zrI0yhOD64E1Dj~la7vTxHLbd1XYrHkSfZecNy-~N-Vxj0QcoamV4R376wrmIRLLZjaPUneJpvW5xz5NZprQPBXzpHhnG84EOx5tn5jkXJMdd~xC3KMTZ5i7foo97Y~55JTLbXnYE8aLJ8UetAAQgNAEMVd9d5xEhLeWOOjsKdjQX5vHNW1YDhg64~e~LIM3fj1IAuFq9DvHApCEQwjowuwKi8qNIKTBjaU2nm8UyyW3WjkledL1P6E~yxI1J4XsG5UuEK~TAugihiQ1C6i8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Mast-cell accumulation and skin morphology after local PMA treatment. Six weeks after treatment of ears with solvent (acetone) (A,C,E,G) or with PMA (B,D,F,H) ear sections were stained with toluidine blue to assess the overall skin morphology and to obtain mast cell counts. Strongly increased skin thickness is apparent in all PMA-treated ears (B-H). Arrows indicate metachromatically stained mast cells in PMA-treated ears. Mast cells accumulated exclusively in the ears of Kit+/+ (B) and KitW/Wv (D) and R−γ−KitW/Wv (F) mice. Mice used were 8 weeks (Kit+/+ [WB]), 11 weeks (KitW/Wv), 25 weeks (R−γ−KitW/Wv), and 13 weeks (KitW/W) of age. The skin of KitW/W mice that lack all Kit cell-surface expression was completely devoid of mast cells. This was true before (G) and after (H) PMA-induced inflammation. The scale bar (A) applies to all panels and corresponds to 40 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-08-039131/4/m_zh80120701220004.jpeg?Expires=1765150083&Signature=0kslPdNGdZnKUOdXGhAbrxHdABNY0dj25w7zFzJhsMl3Ci4a~FugTaJwN~m~SdVms1GELzcTRSakIMg~wtPOV3e1w6wPfDCfPAnHgGk9KuStlKTkfTOZs76CJ~hOXF7i0P4BKsG3GX0jvfOOdb1kPEuM8CXUhrqNPD3rYPldhm-eKeTGLw3Fs~jIrBcPK~xYnmAt998WBH~shXfBh25CmENMn2TUZe~PDRO2PMg4BcZnHzmafRt8OR4mPYB4EkcCMmrwBc3KVyYzGaLrKzndJPvQFl0v8jaho1r5HVn7jzqBzZChThEc7fMas4U2iuvymXkovdlWdG2Itj3~HA4hvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Steady state and inflammation-induced mast cell numbers in the skin of Kit+/+, KitW/Wv, R−γ−, R−γ−KitW/Wv, and KitW/W mice. After 6 weeks of PMA-induced chronic dermatitis, numbers of metachromatic mast cells were determined on toluidine blue–stained sections of ear skin. Closed symbols represent control (acetone)–treated ears, and open symbols represent PMA-treated ears. The figure summarizes data from 2 independent experiments with 3 mice per genotype and experiment (total number of mice = 6). Horizontal lines indicate the mean cell number for each group of mice (A-E). Numbers in healthy skin and the inflammation-induced increase were comparable in Kit+/+ (A) and R−γ− (C) mice. Numbers in healthy skin were very low in untreated KitW/Wv (B) and R−γ−KitW/Wv (D) skin. In PMA-treated skin, the increase in mast cell numbers was approximately 10-fold larger in KitW/Wv compared with R−γ−KitW/Wv mice. In the skin from KitW/W mice, mast cells were undetectable and remained absent after PMA-induced chronic dermatitis (E). The differences in mast cell counts comparing untreated (closed symbols) and PMA-treated (open symbols) skin samples had significant one-sided P values (< .05) (Mann-Whitney test) in panels A through C. The P value (.09) in panel D was not significant. Data from mice of 2 independent experiments were pooled in this figure. Mice used in these experiments were 13 to 21 weeks (Kit+/+ [3 × C57BL/6; 3 × WB]), 8 to 19 weeks (KitW/Wv), 10 to 19 weeks (R−γ−), 9 to 16 weeks (R−γ−KitW/Wv), or 9 to 36 weeks (KitW/W) of age.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-08-039131/4/m_zh80120701220005.jpeg?Expires=1765150083&Signature=WBBCJsFLdqwFOHTMmb3WSU4mfgU8YUyiHVfUNIPHQjn8rZlfC~yEjL1xJYEwKOL~8hPmaEaiogubAjewgSa5-5dyxsxD8y4RzmdqGGG84w6mjZwCH6JJGs4ebJupgapbCBJRRY~h0kAJGpSwQYr5ggkIl2s8YJHBn8XmnEQLQrn~dhpkYIQD7IxFgpfmMUOh18GL3IqbKXNaFCHqJbE9XMoM80Z1gA-dcJg0-aGei2dq-m33ZzB6UH3zMqw7zi9s2qwTRJe3FTkFvxbjSb5Ng36rkC389ul1Bb1Bu5jfay2C8dZwRreZ9~NJOH01EoMmVVvyixff0R2-HqCMdJqWOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. Peritoneal and skin mast cells in Kit mutant mice. Peritoneal exudate cells (PECs) (A-P) and skin (Q-T) from Kit+/+ (A,E,I,M,Q), KitW/Wv (B,F,J,N,R), R−γ−KitW/Wv (C,G,K,O,S), and KitW/W (D,H,L,P,T) mice were analyzed for the presence of mast cells. PEC cytospins were stained with toluidine blue (A-D) and berberine sulfate (E-H). Absence of mast cells in all Kit mutants is evident by lack of staining with these dyes. Scale bars in A and E correspond to 60 μm. (I-L) Flow cytometric analysis of PECs for expression of the mast cell markers T1 and FcϵRI. T1+FcϵRI+ cells were absent in all Kit mutants. Cells on the diagonal, present in all plots, are myeloid cells that are unaffected by mutations in Rag-2, γc, or Kit. Cells on the bottom of the dot plots are lymphocytes that are absent in R−γ−KitW/Wv owing to the mutations in Rag-2, and γc (M-T). Mice were 12 to 13 weeks of age. RT-PCR analysis for expression of mast cell carboxypeptidase A (Mc-cpa), a mast cell–specific gene, as a sensitive tool to detect mast cells in PECs (M-P) and ear skin (Q-T). The triangles indicate 5 cDNA dilutions in 1:10 steps. In line with the analysis shown in panels A through L, PECs lacked Mc-cpa expression in all Kit mutants (N-P). In contrast, Mc-cpa expression was detectable in KitW/Wv (R) and R−γ−KitW/Wv (S) mice. This expression was 10- to 100-fold reduced compared with Kit+/+ skin (Q). Mc-cpa expression was undetectable in the skin of KitW/W mice (T). Mice were 24 weeks (Kit+/+ [C57BL/6]), 12 weeks (KitW/Wv), 31 weeks (R−γ−KitW/Wv), and 17 weeks (KitW/W) old.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-08-039131/4/m_zh80120701220002.jpeg?Expires=1765260555&Signature=UJv38~PUQ-DcCcqRrJW-zvqIVSH8FBgiAsV9~ipcDm2FJqrBEtXMc82ZLqzAIWymU4M2QpWsAb2HwkGREA5WY09o--speSFQtAtWuN9TLV~xMcdou1IKKkb9WSAfzdY8b1y9nNXpkQy5lhMbokJFk1WZJyYFBRdEym027nvqG8FjdZM8OUWWnIJOT0E3WYjH0L~7TE-YUXH39COq67gOsplGc5QNlFeBpHedC-5SzT2koHXiX9iXMNEUPudmwUfGeCy0yusVCLfZkO8vb97QjsYocAu9GWebKz~SB-b8LF82vbfsfAtytjC2F1hxy5EqwoX2PQkr30MJ2pgq6ucqBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Ear swelling during PMA treatment. Ear thickness of PMA-treated ears (■) and solvent-treated contralateral ears (▴) was monitored with a caliper over a period of 6 weeks. Means and SDs of 3 mice per genotype are shown. Data are representative for 1 of 2 complete and independent experiments. Mice used were 13 weeks (Kit+/+ [C57BL/6]), 8 weeks (KitW/Wv), 10 weeks (R−γ−), 16 weeks (R−γ−KitW/Wv), 2 × 9 weeks, and 1 × 16 weeks (KitW/W) old.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-08-039131/4/m_zh80120701220003.jpeg?Expires=1765260555&Signature=SSvOss2tzA8r-SxoptL~1cow-UorOLjIXhLrFjWwVncJLuIkgBqd-yjLMFo9OYXfZcLt2GVEtDgEX8SOhcmm-74YrxAbUJDzGCawv0Mtiq0QP9hgGNNNM2ilgIprt5l2kR9nRwhB5ZfRENP-cNJ-Rj66TyS1LVPDK6AhrLa1bW7II0pNz073fb40Ipa0bVUOSs-l1yIyo-7xu5Bvhyux4WhPNNWhL79C4fG8OuQT-nTg2vFAKuiwKxF24jW6W8rl6D1fW8JJwZC0v0hYD5~2yRI0tUPofaBFrom0L330qiNoYj5-kxOJ05NG1S~~96c6EzMelJWo12tqBZILEnvbVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Mast-cell accumulation and skin morphology after local PMA treatment. Six weeks after treatment of ears with solvent (acetone) (A,C,E,G) or with PMA (B,D,F,H) ear sections were stained with toluidine blue to assess the overall skin morphology and to obtain mast cell counts. Strongly increased skin thickness is apparent in all PMA-treated ears (B-H). Arrows indicate metachromatically stained mast cells in PMA-treated ears. Mast cells accumulated exclusively in the ears of Kit+/+ (B) and KitW/Wv (D) and R−γ−KitW/Wv (F) mice. Mice used were 8 weeks (Kit+/+ [WB]), 11 weeks (KitW/Wv), 25 weeks (R−γ−KitW/Wv), and 13 weeks (KitW/W) of age. The skin of KitW/W mice that lack all Kit cell-surface expression was completely devoid of mast cells. This was true before (G) and after (H) PMA-induced inflammation. The scale bar (A) applies to all panels and corresponds to 40 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-08-039131/4/m_zh80120701220004.jpeg?Expires=1765260555&Signature=KdMoU7Q7wHZB8SJ4XylwtbAw6-d-7dMqZjOzA5USwrBNsNvUScU9p7~RKgYsfQ98Zl2Sjrgto-RuXbdQPPiGmOuq-U48NFHxaCt96BTdyTJeBasUfbhz1W-OA2HCFfqqqcALmU1edIjSxXRrrz0iC5oiUSWYG5K-OOF3UeyXnW4jnOs75x0AcCoFL~btXTNBctf7wJbd4Yqw8uB03wn67fOuZnpGimbcfqkh7mNTr9i8D9CRb2jerd4pvx-Z3iBiTQvoA7KnwxvNSfyf8ToxTfzSrHs06I-fpvBRxlwCyO3jjRQVqjilhYskZBjuwIUBtJ5sV6~PPmo0MFlcX5JNKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Steady state and inflammation-induced mast cell numbers in the skin of Kit+/+, KitW/Wv, R−γ−, R−γ−KitW/Wv, and KitW/W mice. After 6 weeks of PMA-induced chronic dermatitis, numbers of metachromatic mast cells were determined on toluidine blue–stained sections of ear skin. Closed symbols represent control (acetone)–treated ears, and open symbols represent PMA-treated ears. The figure summarizes data from 2 independent experiments with 3 mice per genotype and experiment (total number of mice = 6). Horizontal lines indicate the mean cell number for each group of mice (A-E). Numbers in healthy skin and the inflammation-induced increase were comparable in Kit+/+ (A) and R−γ− (C) mice. Numbers in healthy skin were very low in untreated KitW/Wv (B) and R−γ−KitW/Wv (D) skin. In PMA-treated skin, the increase in mast cell numbers was approximately 10-fold larger in KitW/Wv compared with R−γ−KitW/Wv mice. In the skin from KitW/W mice, mast cells were undetectable and remained absent after PMA-induced chronic dermatitis (E). The differences in mast cell counts comparing untreated (closed symbols) and PMA-treated (open symbols) skin samples had significant one-sided P values (< .05) (Mann-Whitney test) in panels A through C. The P value (.09) in panel D was not significant. Data from mice of 2 independent experiments were pooled in this figure. Mice used in these experiments were 13 to 21 weeks (Kit+/+ [3 × C57BL/6; 3 × WB]), 8 to 19 weeks (KitW/Wv), 10 to 19 weeks (R−γ−), 9 to 16 weeks (R−γ−KitW/Wv), or 9 to 36 weeks (KitW/W) of age.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-08-039131/4/m_zh80120701220005.jpeg?Expires=1765260555&Signature=qXG8A63qebMQ1idRL5GBk8KcZdYqGE4Qeu6WWF6hyyzqf5Rlqa0bJx2RFJp9So~vU8mttUF75jYKBIbZ2Iy0f0IbgjMyFsHAXnMV7gw5BderrDoZgrEPqzULnfM2inNn2mpSWtepiIEvq4EoQ2IYtA0uwm-RhV3YkxYsXjV7efZ-6hdVlcaGhIWkPntNz8OeIfygTcM2-O7jupnTRlfh9qkSObqscme1xJEmBXE70ZCeS3pAfC~RBz-fl1c4RXU50x2MGSAdoBtUClMdsWw~tT2yaGU-nBu9J6bB6WAPj0cGC1Jpf7Ymqc4~mezILrup4kpUnFEhUqHcFOgRwCQOtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
