Abstract
Humanized anti-CD25 antibodies (eg, daclizumab) have been successfully used to treat several autoimmune diseases. Paradoxically, IL-2 blockade in mice can induce autoimmunity. An interspecies difference in the relative contribution of IL-2 to CD25+ T regulatory cell (CD25+Treg) versus CD25+ effector cell function might explain this conundrum. Consistent with this are reports that daclizumab inhibits human CD25+ effector cell cytokine production by blocking the expression of CD40L. However, in mice, IL-4 and IL-12 regulate CD40L expression. As human Th1/Th2 cytokine production is also dependent on IL-2, daclizumab's inhibition of CD40L expression could be due to an indirect, rather than a direct, effect of IL-2. Here, we clarify the mechanisms underlying CD40L expression. In contrast to the mouse, human CD40L is regulated by CD28 signaling and IL-2, not the principal Th1/Th2-polarizing cytokines. We find that CD40L is expressed on naive and memory cells and inhibited by daclizumab independently of cell division. Collectively, our results indicate that daclizumab could inhibit CD25+ effector T-cell function in vivo by directly blocking CD40L expression. This difference between mice and human may help explain the paradoxical effects of IL-2R blockade in the 2 species.
Introduction
Monoclonal antibodies (mAb) directed against cytokines, cytokine receptors, adhesion molecules, and costimulatory ligands are an emerging therapy in both transplantation and autoimmune disease.1,2 Recent reports have described the successful use of a humanized anti-CD25 mAb (daclizumab) in the treatment of several autoimmune diseases.3–11 Daclizumab prevents binding of IL-2 to its high-affinity receptor and subsequent signaling but does not cause depletion of CD25+ cells. The clinical utility of such a mAb was not apparent a priori as the generation and function of CD25+CD4+ T regulatory cells (CD25+Tregs), which are thought to be indispensable for immune tolerance, are reportedly IL-2 dependent.12–14 In fact, anti–IL-2/IL-2R antibodies can induce autoimmunity in mice.14–16 This paradox raises important questions about how daclizumab exerts its clinical efficacy and what the requirements are for CD25+Treg function in humans. Understanding the mechanistic basis of daclizumab's actions should provide critical insights into the relative contribution of IL-2R signaling to CD25+Treg and CD25 effector cell function and their roles in maintaining human immune homeostasis
CD40 ligand (CD40L; CD154) is a well-recognized costimulatory molecule that is expressed on CD4+ T cells shortly after activation. This early expression of CD40L appears to require only a TCR signal. More recently, it has been demonstrated that there is a second, CD28-dependent phase of expression at 48 hours and that this biphasic pattern of CD40L expression is essential to its physiologic function.17,18 While early interaction of CD40L with its receptor (CD40) on B cells initiates a program of B-cell activation, Ig secretion, isotype switching, and B-cell memory formation, sustained CD40L/CD40 signaling inhibits these same processes.17,19 Similarly, CD40L/CD40 interactions on antigen-presenting cells play an important role in promoting T-cell activation and Th1 differentiation, however, constitutive expression is associated with the development of T-cell lymphoproliferative abnormalities.20,21
Late CD40L expression in activated peripheral blood mononuclear cell (PBMC) cultures is almost completely inhibited by daclizumab, leading to the supposition that the CD28 effect on CD40L is mediated through IL-2R signaling. However, this assumption is challenged by contradictory data in the mouse that IL-2 has no effect on CD40L expression. Rather, IL-4 and IL-12 are reported to counterregulate the late phase of CD40L expression with IL-4 inhibiting and IL-12 promoting expression. Because IL-2 receptor blockade also profoundly inhibits both Th1 and Th2 cytokine production in PBMC cultures, the effect of daclizumab on human CD40L expression could be direct or mediated indirectly through IL-4 and IL-12 as reported in the mouse.18 It was also unclear whether early- and late-phase CD40L might represent differential expression on naive and memory CD4 T-cell subsets or be a consequence of cell division.
Here, we address the Th1/Th2 cytokine dependence of human CD40L expression and examine expression on naive and memory cells and the relationship of that expression to CD28 costimulation, cell division, and IL-2R signaling. We observe fundamental differences in the regulation of CD40L between mice and humans, a finding that has important implications for multiple models of immune function and disease. The presented data provide information that is important to understanding the contribution of IL-2 to effector T-cell function. The direct inhibition of CD40L expression by daclizumab that we describe here, and the resulting inhibition of CD40L/CD40 effector functions we reported earlier, suggests that this mechanism could play a vital role in the clinical activity of IL-2R–blocking mAbs. The clinical efficacy of daclizumab in multiple autoimmune diseases and the in vitro results reported here suggest that in vivo inhibition of CD25+ effector T-cell function may be more clinically relevant than any inhibition of CD25+ Tregs that occurs.
Patients, materials, and methods
Subjects
Buffy coat fractions were obtained from leukopheresed subjects belonging to the National Institutes of Health healthy adult donor pool (IRB-approved protocol no. 99-CC-0168). Umbilical cord blood was obtained through the National Institutes of Health cord blood program (IRB-approved protocol no. 01-DK-0122). All specimens were processed and plated into the appropriate cultures within 18 hours of collection.
Cell culture reagents
Complete media (Biosource, Rockville, MD) consisting of RPMI 1640 supplemented with penicillin (100 U/mL), streptomycin (100 U/mL), l-glutamine (2 mM), and 10% fetal bovine serum (FBS; Hyclone, Logan, UT) was used for all stimulations. Lymphocyte separation media were purchased from Mediatech (Herndon, VA). Ficoll-Paque was from Amersham Biosciences (Uppsala, Sweden).
Media used for Th1 versus Th2 cell polarization
All CD4+ cells were cultured in X-Vivo 20 media supplemented with 5% human AB serum (Gemini Bioproducts, Woodland, CA). The Th1 culture condition included the following: rhuIL-12 (2.5 ng/mL; Peprotech, Rocky Hill, NJ); rhuIL-2 (1000 IU/mL; Chiron, Emeryville, CA); and anti–IL-4 antibody. Murine antihuman IL-4 antibody was generated from the B-cell hybridoma, MP4.25D2.11 (ATCC, Manassas, VA); the hybridoma was grown in serum-free X-Vivo 20 media, and the supernatant was collected and used without further purification. The supernatant was tested for its ability to compete for recombinant human IL-4 (rhuIL-4) in an enzyme-linked immunosorbent assay (ELISA; BioSource, Camarillo, CA), and an amount of supernatant sufficient to neutralize 6 μg/mL rhuIL-4 was added to the Th1 cultures. The Th2 culture condition included rhuIL-4 (1000 IU/mL; Peprotech) and rhuIL-2 (20 IU/mL).
Recombinant proteins in PBMC cultures
Human rIL-4 and rIL-12 were purchased from R&D Systems (Minneapolis, MN) and rIL-2, from Roche (Indianapolis, IN).
Antibodies in PBMC cultures
Humanized anti-Tac (HAT; anti-CD25; daclizumab) was a generous gift from Hoffmann-La Roche (Nutley, NJ). Antibodies to CD28 (clone CD28.2), CD80 (clone 307.4), CD86 (clone IT2.2), IL-12Rβ1 (clone 2.4E6), IFN-γ (clone B27), IFN-γRα (clone GIR-208), and IL-4 (clone MP4-25D2) were purchased from BD Pharmingen (San Diego, CA), as were the isotype controls. Anti–IL-12 antibody was purchased from R&D Systems. OKT3 was obtained from Ortho Biotech (Raritan, NJ).
PBMC fractionation
PBMCs were isolated from leukopheresed buffy coat fractions by density gradient centrifugation using lymphocyte separation media. Purified CD4+ T cells were isolated directly from buffy coats using the RosetteSep antibody cocktail (StemCell Technologies, Vancouver, BC) according to the manufacturer's directions. Antihuman CD45RO and CD45RA MicroBeads (Miltenyi Biotec, Auburn, CA) were used for negative selection of CD45RA+ and CD45RO cells, respectively, from purified CD4+ T cells on an AutoMacs instrument (Miltenyi Biotec). Antihuman CD3 MicroBeads (Miltenyi Biotec) were used to deplete PBMCs of T cells on an AutoMacs instrument. Depletion of these cells was more than 95%. Purified CD4+ T cells were added back to CD3-depleted PBMCs in the same proportion as they were originally present in the PBMCs.
Isolation of CD4+ T cells for Th1/Th2 polarization
B cells and CD8+ T cells were depleted by 2 rounds of negative selection using anti-CD20 and anti-CD8 murine antihuman antibodies (Nexell, Irvine, CA) in combination with sheep antimouse magnetic beads using an Isolex device (Nexell). The enriched CD4+ T cells, which were more than 95% pure by flow cytometry (< 0.1% CD8+ T-cell contamination), were then cryopreserved under liquid nitrogen conditions in X-Vivo 20 media (BioWhittaker; Walkersville, MD) containing 5% DMSO, 5% pentastarch, and 40% human serum albumin.
PBMC stimulations with plate-bound anti-CD3
PBMCs (1 × 106 cells/mL) in complete media were added to flat-bottom 6-well (4 to 8 mL) or 24-well (2 mL) tissue culture plates (Costar, Cambridge, MA) and stimulated with immobilized OKT3 (plates previously coated at room temperature overnight at 2 μg/mL) ± soluble anti-CD28 antibody at 2 μg/mL in the presence of various cytokines and/or antibodies. Additional antibodies were used at the following final concentrations based on the suppliers' recommendations and our own titration data: antihuman IL-12 (2 μg/mL), IL-12Rβ1 (0.5 μg/mL), IFN-γ (1 μg/mL), IFN-γRα (5 μg/mL), IL-4 (4 μg/mL), and CD25 (daclizumab, 10 μg/mL). The final concentration of recombinant cytokines used is indicated in the results. Trypan blue staining of the cultures indicated that under all stimulation conditions there was no change in viability (> 80%) over the period of the experiments.
CD3, CD28 bead-based CD4+ T-cell expansion
At culture initiation, frozen CD4+ T cells were thawed, and stimulated with tosylated magnetic beads (Dynal, Oslo, Norway) conjugated with antihuman CD3 (OKT3) and antihuman CD28 (clone 9.3) antibodies at a cell to bead ratio of 1:3. Antibody-coated beads were prepared as previously detailed.22 Initial T-cell concentration was 0.3 × 106 cells/mL; cell concentration and volume were measured by Coulter Multisizer II (Beckman Coulter, Fullerton, CA). Cell density was maintained below 1 × 106 cells/mL by addition of cytokine-replete Th1- or Th2-specific media; however, IL-12 was added to Th1 cultures at culture initiation only. On day 12 of culture, CD4+ cells were restimulated with anti-CD3, anti-CD28–coated beads in cytokine-replete media (cell to bead ratio, 1:3).
Flow cytometric analysis
Flow cytometric analysis was performed according to standard protocols. Fluorochrome-labeled anti-CD4 (APC), anti-CD40L (PE), and isotype control antibodies were purchased from BD Pharmingen. Cells were stained with the appropriate antibodies according to the manufacturer's suggestions. Viaprobe (BD Biosciences) was added to gate out dead cells. All incubations were performed at 4°C in staining buffer, and cells were washed twice and fixed in 1% paraformaldehyde in PBS. Stained cell populations were acquired using a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA). Data were analyzed using CellQuest (Becton Dickinson). Color compensation settings were made with each round of staining using appropriate fluorochrome-conjugated antibodies.
Statistical analysis
The nonparametric rank-sum analysis of Wilcoxin was used to determine the P value in those experiments (Figure 6A) where the number of donors was sufficiently large, otherwise, a paired t test was performed.
Results
rIL-2 restores CD40L expression in CD80/86 blocked cultures
Earlier studies had demonstrated that IL-2R signaling is required for late CD40L expression but had not ascertained whether CD28 signaling is itself necessary. To address this question, we asked if rIL-2 could substitute for CD28 costimulation. As CD28 is constitutively expressed on CD4+ T cells and PBMCs express variable levels of the CD28 ligands CD80 and CD86, we performed these experiments in the presence of blocking anti-CD80 and anti-CD86 mAbs. As a control, we confirmed that the blocking mAbs did not have inhibitory effects other than those due to disrupting interactions with CD28. As shown in Figure 1A, the blocking mAbs to CD80/86 had no effect on CD40L expression in the presence of anti-CD28 mAb, while daclizumab severely inhibited CD40L expression after 6 hours. By contrast, in PBMC cultures stimulated with anti-CD3 alone, blocking CD80/86 inhibited CD40L expression after 6 hours (Figure 1B). Addition of rIL-2 to CD80/86-blocked cultures restored CD40L expression to the level seen on CD28-costimulated cells (Figure 1B). These results demonstrate that CD28 signaling affects CD40L expression indirectly and that the effect of CD28 costimulation on CD40L expression is wholly mediated through its augmentation of IL-2 production.
CD28-dependent CD40L expression is mediated via IL-2R signaling. (A) CD28-costimulated PBMCs were cultured with anti-CD80/86 or anti-CD25 mAb. (B) TCR-stimulated PBMCs were cultured with anti-CD80/86 mAb ± rIL-2. rIL-2 was added at 24 hours. All control cultures were incubated with the appropriate isotype mAb. Gating was performed on CD4+ lymphocytes. The means ± SE of 4 donors are shown.
CD28-dependent CD40L expression is mediated via IL-2R signaling. (A) CD28-costimulated PBMCs were cultured with anti-CD80/86 or anti-CD25 mAb. (B) TCR-stimulated PBMCs were cultured with anti-CD80/86 mAb ± rIL-2. rIL-2 was added at 24 hours. All control cultures were incubated with the appropriate isotype mAb. Gating was performed on CD4+ lymphocytes. The means ± SE of 4 donors are shown.
Both naive and memory cells exhibit biphasic CD40L expression
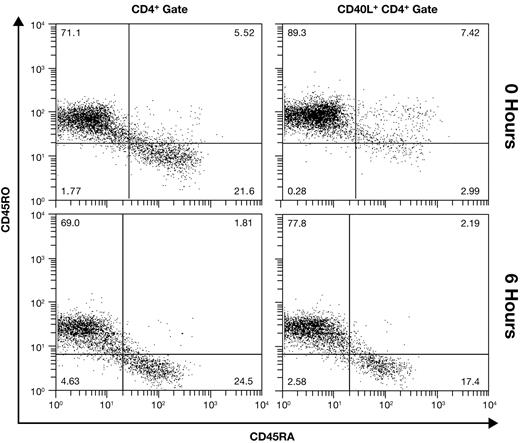
Adult PBMCs contain both naive (CD45RA+CD45RO−) and memory (CD45RA−CD45RO+) CD4+ T cells. Memory cells are generally considered to have a lower threshold for activation, possibly diminishing or negating their need for a costimulatory signal. We thus hypothesized that CD28-independent CD40L at 6 hours may reflect expression on activated memory cells, while CD28-dependent late expression occurs on naive cells. We initially addressed this question in unfractionated PBMCs using 4-color flow cytometry. Results from a donor with approximately 70% CD45RO+CD4+ cells are shown in Figure 2. In unstimulated PBMCs (0 hours), gating on CD40L+CD4+ cells (1.6% of CD4+ lymphocytes) reveals that expression is almost entirely on memory cells. However, at 6 hours after activation, CD40L is expressed proportionately on naive and memory cells (34.2% of CD4+ lymphocytes), indicating that naive and memory cells have a nearly equivalent capacity to express CD40L early.
Equivalent expression of early CD40L on naive and memory cells. The memory phenotype of CD4+ cells (left panels) and CD40L+ CD4+ cells (right panels) was differentiated in unfractionated resting (0 hour) and CD28-costimulated PBMCs using 4-color flow cytometry. Results from a representative donor are shown.
Equivalent expression of early CD40L on naive and memory cells. The memory phenotype of CD4+ cells (left panels) and CD40L+ CD4+ cells (right panels) was differentiated in unfractionated resting (0 hour) and CD28-costimulated PBMCs using 4-color flow cytometry. Results from a representative donor are shown.
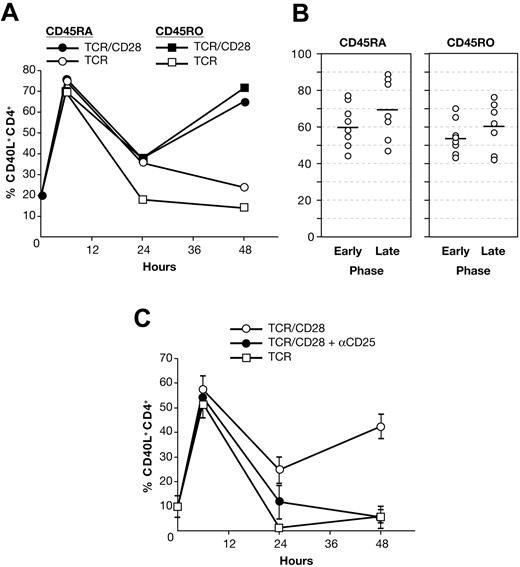
To assess the memory phenotype of CD40L+ cells at later time points, CD4+ T cells were separated into pure CD45RO and CD45RA single-positive populations. To recapitulate the PBMC culture milieu, we reconstituted CD3-depleted PBMCs with either pure CD45RO+ or CD45RA+ CD4+ T cells prior to stimulation. As shown in Figure 3A, both activated CD45RO+ and CD45RA+ cells exhibit biphasic CD40L expression. While in this donor, CD45RO+ and CD45RA+ cells express CD40L equally, on average, expression was slightly greater on CD45RA+ cells at early and late time points (Figure 3B). These findings indicate that naive and memory cells have a nearly equivalent capacity to express CD40L and that late-phase expression on both is CD28 dependent.
Naive and memory cells both express CD40L early and late. (A) CD40L expression in stimulated, reconstituted PBMC cultures of pure CD45RA+ or CD45RO+ CD4+ T cells from a single donor. (B) Early- and late-phase CD40L expression in reconstituted PBMCs from 13 donors. Due to overlap of the data points, not all symbols are visible. Solid bars indicate the mean. (C) Umbilical cord blood mononuclear cells were stimulated ± anti-CD25 mAb. The means ± SE of 4 donors are shown.
Naive and memory cells both express CD40L early and late. (A) CD40L expression in stimulated, reconstituted PBMC cultures of pure CD45RA+ or CD45RO+ CD4+ T cells from a single donor. (B) Early- and late-phase CD40L expression in reconstituted PBMCs from 13 donors. Due to overlap of the data points, not all symbols are visible. Solid bars indicate the mean. (C) Umbilical cord blood mononuclear cells were stimulated ± anti-CD25 mAb. The means ± SE of 4 donors are shown.
To confirm that naive cells exhibit biphasic CD40L expression, we examined CD40L expression in umbilical cord blood, the only bona fide source of naive human T cells. Unfractionated umbilical cord blood mononuclear cells, consisting of more than 95% CD45RA+CD45RO− CD4+ lymphocytes, were stimulated under the same culture conditions used for adult PBMCs. As shown in Figure 3C, activated neonatal CD4+ cells express CD40L at early and late time points, and such expression exhibits the same pattern of CD28 and IL-2 dependence as that shown by adult PBMCs. This temporal pattern of expression is consistent with the report that naive mouse CD4+ T cells exhibit biphasic CD40L expression with the same kinetics as PBMCs.17
Daclizumab blocks CD40L expression independently of its inhibition of Th1/Th2 cytokine production
CD40L is important for both humoral and cellular immune responses and thus it is plausible that Th1 and/or Th2 cytokines may play a role in either positively or negatively regulating its expression. In the mouse, IL-4 and IL-12 counterregulate the late phase of CD40L expression with IL-4 inhibiting and IL-12 promoting expression, but, remarkably, IL-2 is reported to have no effect.17 Daclizumab abolishes late CD40L expression and profoundly inhibits both Th1 and Th2 cytokine production in CD28-costimulated PBMCs.18 Thus IL-2 could be acting directly to promote late human CD40L expression or indirectly through the action of IL-2–dependent cytokines such as IL-4, IFN-γ, and IL-12, as reported in the mouse.17 To distinguish between these possibilities, we first added to PBMC cultures anti–IL-4 mAb sufficient to neutralize 10 ng/mL rIL-4. We had previously found that activated PBMCs produce 0.02 to 0.10 ng/mL IL-4 at 48 hours. Despite the use of a 100-fold excess of IL-4–neutralizing mAb, there was no effect on CD40L expression (Figure 4A). This demonstrates that IL-4 in situ does not regulate early or late CD40L expression. In mouse T-cell cultures, addition of rIL-4 is reported to inhibit late CD40L expression; so we tested if high-dose rIL-4 could alter human CD40L expression.17 Addition of 25 ng/mL rIL-4, a concentration 250-fold greater than that observed in situ, also had no impact on the number of CD40L-expressing cells (Figure 4B) or the level (MFI) of CD40L expression (Figure 4C).
Biphasic CD40L expression is IL-4 independent. PBMCs were stimulated with OKT3 ± anti-CD28 mAb. Gating was performed on CD4+ lymphocytes. (A) Anti–IL-4 or isotype control mAb was added to parallel cultures. (B-C) Costimulated PBMCs were cultured ± rIL-4. The mean ± SE of 4 donors are shown. (D) Anti–IL-12 and anti–IL-12R mAb or isotype controls were added to parallel cultures. The mean ± SE of 3 donors are shown. (E) Stimulated PBMC cultures ± rIL-12. The means ± SE of 5 donors are shown. (F) Stimulated PBMC cultures ± rIL-12 ± anti-CD25 mAb. The mean ± SE of 5 donors are shown.
Biphasic CD40L expression is IL-4 independent. PBMCs were stimulated with OKT3 ± anti-CD28 mAb. Gating was performed on CD4+ lymphocytes. (A) Anti–IL-4 or isotype control mAb was added to parallel cultures. (B-C) Costimulated PBMCs were cultured ± rIL-4. The mean ± SE of 4 donors are shown. (D) Anti–IL-12 and anti–IL-12R mAb or isotype controls were added to parallel cultures. The mean ± SE of 3 donors are shown. (E) Stimulated PBMC cultures ± rIL-12. The means ± SE of 5 donors are shown. (F) Stimulated PBMC cultures ± rIL-12 ± anti-CD25 mAb. The mean ± SE of 5 donors are shown.
Having ruled out involvement of IL-4, we assessed the possible contribution of the Th1-polarizing cytokine IL-12. On average, CD28-costimulated PBMCs produce 25 pg/mL IL-12p70 at 48 hours. When IL-12–neutralizing and IL-12R–blocking mAbs sufficient to completely neutralize 100 pg/mL rIL-12 (or inhibit 750 pg/mL by 50%) were added to such cultures, there was no effect on CD40L expression (Figure 4D). Parallel experiments to assess the role of IFN-γ demonstrated that, as in the mouse, IFN-γ has no impact on human CD40L expression (data not shown). The addition of rIL-12 has been reported to enhance late CD40L expression in mouse, thus we tested if high-dose IL-12 could alter CD40L expression.17 As shown in Figure 4E, rIL-12 did significantly promote CD40L expression in TCR-stimulated cultures at 48 hours (P = .012) and at 72 hours (P = .004). This effect of rIL-12 was also apparent in the presence of CD28 costimulation at 48 hours (P = .001) and 72 hours (P = .007). However, unlike rIL-2, even 100 ng/mL rIL-12 could not raise the level of CD40L in TCR-stimulated cultures to that observed with CD28 costimulation. Because endogenous IL-12 production in our PBMC cultures is dependent on CD4+ T cells and IL-2, we asked whether the ability of added rIL-12 to promote CD40L expression is itself IL-2 dependent. As shown in Figure 4F, when daclizumab was added to rIL-12–treated PBMCs, CD40L expression was markedly inhibited at 48 hours (P = .013) and 72 hours (P = .03), but not to the level seen in daclizumab-treated cultures without rIL-12 (TCR/CD28/αCD25 vs TCR/CD28/αCD25 + rIL-12, P = .008 at 48 hours). Thus, while endogenous IL-12 production in this PBMC system is itself IL-2 dependent, high-dose rIL-12 can moderately enhance CD40L expression in an IL-2–independent fashion.
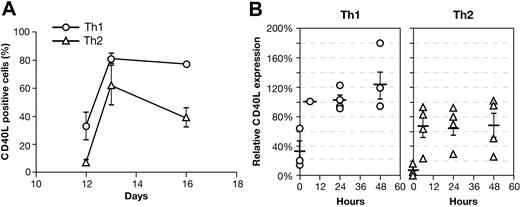
Unlike immunized mice, human PBMCs do not display highly skewed Th1 or Th2 phenotypes. Thus, we also examined CD40L expression on CD4+ T cells expanded under Th1- or Th2-polarizing conditions. After 12 days, cultures were again stimulated in fresh media under neutral or polarizing conditions. Cells restimulated under neutral culture conditions were assayed to confirm they had acquired a polarized phenotype (Figure 5). Cells restimulated under polarizing conditions were assessed for CD40L expression 1 and 4 days later. On day 12, resting Th1-polarized CD4+ T cells have significantly more (P = .03) CD40L than do Th2 cells, which expressed baseline levels of CD40L (Figure 6A). However, upon restimulation, both Th1- and Th2-polarized CD4+ T cells abundantly express CD40L (day 13). By day 16, while CD40L remains elevated on Th1- and Th2-polarized CD4+ T cells, there was again significantly more CD40L on Th1 cells (P = .001). Of interest, day-12 polarized CD4+ T cells restimulated under neutral culture conditions have a pattern of CD40L expression similar to that found on cells restimulated under polarizing conditions (Figure 6B).
Cytokine profile of Th1- and Th2-polarized CD4+ T cells. Purified CD4+ T cells were stimulated on day 0 with bead-adsorbed anti-CD3 and anti-CD28 mAb under Th1- or Th2-polarizing conditions. On day 12 cells were harvested and restimulated for 24 hours with mAb-coated beads under neutral conditions, and culture supernatants were assayed by cytokine ELISA. The mean ± SE of 16 donors are shown.
Cytokine profile of Th1- and Th2-polarized CD4+ T cells. Purified CD4+ T cells were stimulated on day 0 with bead-adsorbed anti-CD3 and anti-CD28 mAb under Th1- or Th2-polarizing conditions. On day 12 cells were harvested and restimulated for 24 hours with mAb-coated beads under neutral conditions, and culture supernatants were assayed by cytokine ELISA. The mean ± SE of 16 donors are shown.
CD40L expression is skewed on polarized Th1/Th2 cells. Purified CD4+ T cells were stimulated on day 0 with bead-adsorbed anti-CD3 and anti-CD28 mAb under Th1- or Th2-polarizing conditions. (A) On day 12, CD40L expression was measured and cells were restimulated as on day 0 (n = 16). CD40L expression was measured again on day 13 (n = 16) and day 16 (Th1 n = 5; Th2 n = 16). The mean ± SE is shown. (B) CD40L expression was measured at 0 hours (day 12) and following restimulation under the same nonpolarizing conditions used for PBMC cultures. Data are expressed relative to the 6-hours Th1 sample. Due to overlap of the data points, not all symbols are visible. Solid bars indicate the mean (n = 4). Standard error bars are shown.
CD40L expression is skewed on polarized Th1/Th2 cells. Purified CD4+ T cells were stimulated on day 0 with bead-adsorbed anti-CD3 and anti-CD28 mAb under Th1- or Th2-polarizing conditions. (A) On day 12, CD40L expression was measured and cells were restimulated as on day 0 (n = 16). CD40L expression was measured again on day 13 (n = 16) and day 16 (Th1 n = 5; Th2 n = 16). The mean ± SE is shown. (B) CD40L expression was measured at 0 hours (day 12) and following restimulation under the same nonpolarizing conditions used for PBMC cultures. Data are expressed relative to the 6-hours Th1 sample. Due to overlap of the data points, not all symbols are visible. Solid bars indicate the mean (n = 4). Standard error bars are shown.
Daclizumab inhibits CD40L expression independently of cell proliferation
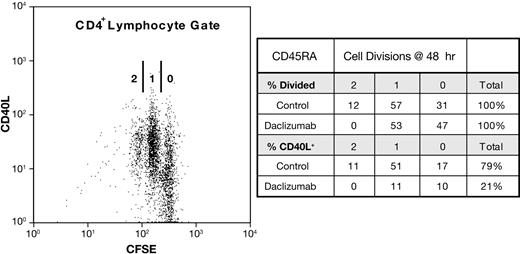
To ascertain whether CD40L expression is coupled to cell division in naive or memory cells, we again used reconstituted PBMCs consisting of either pure CD45RA+ or CD45RO+ CD4+ T cells that had been CFSE labeled. At 6 hours, no CD4+ T cells had divided and thus early CD40L expression is independent of cell division (data not shown). At 48 hours, about half of the CD4+ T cells have divided at least once. The distribution of CD40L on cells that had undergone 0, 1, or 2 cell divisions at 48 hours reflected the donors' proliferative capacity and increased with cell division. This is illustrated by the donor shown in Figure 7, in whom 92% (11%/12%) of CD45RA+ cells that divided twice were CD40L+, whereas 55% (17%/31%) of the cells that had not divided expressed CD40L. In daclizumab-treated cultures, CD40L expression was inhibited on cells that had not divided as well as those that had. Replication beyond one cell division was completely blocked by daclizumab, but the number of cells dividing once was unaffected. By contrast, on cells that had divided once, CD40L expression was inhibited 80%. On average, results for CD45RO+ cells were similar to those obtained with CD45RA+ cells (Tables 1 and 2). These results demonstrate that while late-phase CD40L expression is proportionately greater on cells that have divided, division is not necessary for CD40L expression and inhibition by daclizumab is independent of blocking cell division, establishing that IL-2 affects CD40L expression independently of cell proliferation.
Daclizumab inhibits CD40L expression independently of cell division. Reconstituted PBMC cultures of pure, CFSE-labeled CD45RO+ or CD45RA+ CD4+ T cells were CD28 costimulated ± anti-CD25 Ab. On the left, a dot plot of such a CD28-costimulated culture of CD45RA+ cells at 48 hours is shown. The number of cell divisions (0, 1, 2) as measured by CFSE dilution is indicated. On the right, the distribution of divided cells and CD40L on those cells, in the presence (daclizumab) and absence (control) of anti-CD25 is shown.
Daclizumab inhibits CD40L expression independently of cell division. Reconstituted PBMC cultures of pure, CFSE-labeled CD45RO+ or CD45RA+ CD4+ T cells were CD28 costimulated ± anti-CD25 Ab. On the left, a dot plot of such a CD28-costimulated culture of CD45RA+ cells at 48 hours is shown. The number of cell divisions (0, 1, 2) as measured by CFSE dilution is indicated. On the right, the distribution of divided cells and CD40L on those cells, in the presence (daclizumab) and absence (control) of anti-CD25 is shown.
Discussion
CD40L is an essential molecule involved in promoting B- and T-cell responses, and the consequences of human CD40L deficiency are evident in the X-linked hyper-IgM syndrome.23 It has long been recognized that CD40L is rapidly expressed and down-regulated on CD4+ T cells following TCR engagement.24–27 More recent data from our lab and the Randall lab have shown that both human and mouse CD4+ T cells exhibit a biphasic pattern of CD40L expression, indicating that its regulation and function are more complex than originally envisioned (Lee et al17 and McDyer et al18 ). In the mouse, it has been clearly established that the biphasic character of CD40L expression is critical to its physiologic function.17,19 Human data also support the premise that early- and late-phase CD40L are functionally distinct as CD40L-dependent IL-12 production is completely abolished by inhibiting only the late-phase of CD40L expression.18 Human CD40L expression is biphasic both temporally and mechanistically. There is an initial CD28-independent peak at 6 hours that is followed by a nadir at 24 hours and a second, CD28-dependent peak of expression at 48 to 72 hours. Here, we have expanded upon those preliminary observations by showing that biphasic expression is an integral aspect of CD40L regulation and not a result of cell division or differential expression on naive and memory CD4+ cells. On the contrary, though CD40L expression on resting CD4+ T cells is almost entirely restricted to memory cells, upon activation adult naive and memory CD4+ cells have a nearly equivalent capacity to express CD40L early and late and are equally dependent upon CD28 signaling. While memory cells are widely regarded to be less dependent on costimulation, late-phase CD40L and (by implication) IL-2 expression clearly remain dependent upon a CD28 signal. Although the use of surface markers to distinguish naive and memory cells may never be unequivocal, CD45RA and CD45RO are widely accepted as a means of broadly differentiating these populations. To confirm the capacity of naive CD4+ cells to express biphasic CD40L, rather than rely on additional surface markers (eg, CD11a, CD27, CD62L, CCR7) that disputably distinguish between memory and naive cells, we examined CD40L expression on neonatal CD4+ cells, the only certain source of naive human T cells. While there are many reports that neonatal CD4+ T cells are not fully immunocompetent, we thought that examining CD40L expression on these cells may nonetheless be informative.28–34 Indeed, we found that umbilical CD4+ T cells, like naive adult CD4+ T cells, exhibit early CD28-independent and late CD28-dependent CD40L expression upon T-cell activation. Our results are consistent with the report that primary mouse CD4+ T cells exhibit biphasic CD40L expression with kinetics identical to what we have reported in PBMCs.17
The studies presented here also provide information that is important to understanding the mechanistic basis of late CD40L expression and the contribution of IL-2 to effector T-cell function. The ability of rIL-2 to substitute for CD28 costimulation establishes that CD28 signaling does not directly regulate CD40L expression on human CD4+ T cells and further demonstrates that the CD28-mediated regulation of late CD40L expression is dependent upon IL-2. The level of IL-2 expression in the culture impacts CD40L expression at all times after 6 hours to the extent that in some donors the nadir at 24 hours may not be apparent without blocking IL-2R. While these results show that IL-2 is necessary for late CD40L expression, and we have demonstrated that such expression is not a result of IL-2–driven proliferation, they do not establish that IL-2 directly regulates CD40L expression. Furthermore, though the kinetics of biphasic CD40L expression in mice and humans are identical, IL-2 is reported to have no effect on the expression of mouse CD40L. Rather, in the mouse, IL-4 and IL-12 counterregulate the late phase of CD40L expression with IL-4–inhibiting and IL-12– promoting expression.17 Because Th1 and Th2 cytokine production in PBMC cultures is profoundly dependent upon IL-2, our earlier experiments did not distinguish whether the effect of IL-2 on human CD40L expression was a direct one or mediated indirectly through IL-4 and IL-12 as reported in the mouse.18 While IL-4 has no effect on human CD40L expression, we did find that, as previously reported for purified human T cells, exogenous rIL-12 augments CD40L expression in primary PBMC cultures. While the effect of rIL-12 is partially IL-2 independent, rIL-12 cannot substitute for CD28 or IL-2R signaling. Additionally, CD40L is expressed at a higher frequency and for a sustained period in polarized Th1 CD4+ T-cell cultures relative to their Th2 counterpart. These differences are not dependent on the continued presence of IL-4 or IL-12 (Figure 6B), suggesting that such changes may reflect the differentiation state of these long-term cultured cells. The counterregulation of CD40L expression by IL-4 and IL-12 in the mouse has been interpreted in the context of a model in which late-phase (ie, IL-12 driven) CD40L expression, in contrast to early-phase expression, inhibits B-cell activation and immunoglobulin production.17 These observations in the mouse are in contradistinction to the reports that in humans, rIL-12 promotes B-cell proliferation and immunoglobulin production in a CD40L-dependent fashion.35,36 While we have not attempted to exhaustively rule out a role for other cytokines in regulating CD40L expression, we can conclude that the effect of IL-2 is not mediated through the principal IL-2–dependent Th1/Th2 cytokines and that even pharmacological doses of IL-12 play a subordinate role.
Although Lee et al17 found that IL-2 does not impact mouse CD40L expression, several reports do indicate a role for CD28/IL-2 signaling.17 While early CD40L expression is unaltered in the CD28−/− mouse, B7 blockade in vitro and in vivo has been shown to inhibit CD40L expression, indicating that CD28 signaling plays a role at later time points.37–40 Additionally, CD28−/− mice have been shown to have severely impaired IL-12 production at 48 hours. Correspondingly, in human PBMCs, CD28-mediated up-regulation of IL-12 is dependent upon IL-2R signaling.18,41 Finally, IL-2 and STAT5 in naive mouse T cells have been shown to play an essential role in IL-4 production.42–45 It is unclear how to reconcile these earlier demonstrations of the IL-2 dependence of CD40L, IL-12, and IL-4 expression in the mouse, collectively or individually, with the report that mouse CD40L expression is entirely independent of IL-2 but regulated by IL-4 and IL-12. It is striking that the regulation of such a fundamental molecule as CD40L may be as divergent as has been described in mice and humans, yet there is precedence for genetically encoded immunologic differences between these species.46
Humanized monoclonal antibodies directed against the CD25 subunit of the high-affinity IL-2R have been shown to have clinical utility in several distinct autoimmune entities.3–11 These reports were quite unexpected as IL-2 blockade in mice can induce autoimmune disease, apparently as a consequence of inhibiting CD25+Treg function.14–16,47 The immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX) syndrome, an autoimmune disorder with phenotypic similarities to CD25+Treg deficiency in the mouse, establishes that CD25+Tregs have a significant role in establishing immune tolerance during the major period of human lymphocyte development.48 However, evidence that CD25+Tregs are of functional importance in human adults is lacking.49 Despite the results of the IL-2–blocking experiments in mice, it remains controversial whether extrathymic CD25+Tregs require IL-2 in vivo.50,51 The therapeutic success of daclizumab in autoimmune disorders and our earlier demonstration that it blocks CD28-dependent CD40L expression and CD40L-dependent IL-12 production, as well as drastically reduces IFN-γ production, suggest that inhibition of IL-2–dependent effector mechanisms might explain the apparently paradoxical clinical efficacy of the antibody. Collectively, the data presented here indicate that the inhibitory activity of daclizumab is in all likelihood due to a direct effect of IL-2 on CD40L expression. These findings suggest that human CD25+Tregs in the periphery either do not require IL-2 or that, on balance, inhibition of IL-2R signaling in CD25+CD4+ effector cells is more clinically significant than inhibition of CD25+Treg function.
In summary, understanding the mechanistic basis of daclizumab's inhibitory activity has provided important insights into the regulation of CD40L expression and the contribution of IL-2R signaling to CD25+ effector T-cell function. These findings, and our previous reports that daclizumab inhibits the effects of CD40L/CD40 signaling, in conjunction with the demonstrated clinical efficacy of daclizumab, challenge the notion that in humans the primary function of IL-2 is to promote and sustain CD25+Tregs.52 Additional human data, such as the well-known immunotherapeutic effect of administered IL-2 in melanoma and renal carcinoma, also cast doubt on concepts of CD25+Tregs that have been developed in the mouse.53 Inhibition of IL-2 effector functions, in addition to less well-defined mechanisms such as the expansion of natural killer (NK) regulatory cells, is likely to contribute to the therapeutic effect of daclizumab.54,55 Our findings reveal a potentially fundamental difference between humans and mice in the regulation of CD40L expression that has profound implications for mouse models of B-cell maturation, transplant tolerance, allergy, and autoimmune disease. Beyond their research implications, these differences have direct clinical ramifications as well. Studies of CD40L in the mouse would suggest that anti–IL-12 therapy, which has demonstrated clinical efficacy in autoimmune disease, could act in part by down-regulating CD40L expression.56,57 Such an inference would suggest that anti–IL-12 therapy might represent an alternate means by which to inhibit CD40L function, an immunomodulatory strategy that has been shown to induce long-term graft tolerance in a primate allotransplant model.58 On the contrary, our data in primary human T cells suggest that therapies directed against IL-2, but not IL-12, are more likely to be effective in modulating CD40L expression and might represent an alternative strategy to anti-CD40L mAb-based therapy and its associated adverse effects.58
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC ection 1734.
Acknowledgments
We thank George Reed for assistance with the statistical analysis, Elizabeth Joyal for cord blood collections, and John McDyer, John O'Shea, Robert Seder, and Tom Waldmann for critical reading of the paper.
National Institutes of Health
Authorship
Contribution: J.A.R. conceived and designed the experiments, analyzed and interpreted the data, and wrote the paper; J.T.S., J.S., and H.A. designed and performed experiments, collected data, and analyzed and interpreted results; J.H. and D.H.F. conceived, executed, and analyzed the results of the Th1/Th2 experiments reported in the paper; J.T.S., J.S., and D.H.F. contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jack A. Ragheb, Bldg 10, Rm 10N113, 10 Center Dr, MSC-1857, Bethesda, MD 20892-1857; e-mail: jr50b@nih.gov.