Abstract
Atiprimod is a novel cationic amphiphilic compound and has been shown to exert antimyeloma effects both in vitro and in mouse experiments. This study was undertaken to evaluate the therapeutic efficacy of atiprimod on mantle cell lymphoma (MCL) and elucidate the mechanism by which it induces cell apoptosis. Atiprimod inhibited the growth and induced apoptosis of MCL cell lines and freshly isolated primary tumor cells in vitro. More importantly, atiprimod significantly inhibited tumor growth in vivo and prolonged the survival of tumor-bearing mice. However, atiprimod also exhibited lower cytotoxicity toward normal lymphocytes. Atiprimod activated c-Jun N-terminal protein kinases (JNK) and up-regulated the level of Bax, Bad, and phosphorylated Bcl-2, resulting in release of apoptosis-inducing factor (AIF) and cytochrome c from mitochondria and activation and cleavage of caspase-9, caspase-3, and PARP. However, AIF, but not activation of caspases or PARP, was responsible for apoptosis in MCL cells because an AIF inhibitor, but not pan-caspase or paspase-9 inhibitors, completely abrogated atiprimod-induced apoptosis. Taken together, our results demonstrate that atiprimod displays a strong anti-MCL activity. Cell apoptosis was induced mainly via activation of the AIF pathway. These results support the use of atiprimod as a potential agent in MCL chemotherapy.
Introduction
Mantle cell lymphoma (MCL) represents approximately 8% of all non-Hodgkin lymphomas (NHLs). It has the worst prognosis among all B-cell–derived malignancies with median survival of 3 to 4 years.1–3 Although MCL has been treated with chemotherapy, radiotherapy, hematopoietic stem cell transplantation, molecular targeted therapy, and combination therapy of these treatments, no standard treatment approach has been established thus far. Novel therapeutic agents are needed in clinical trials.4–8 Atiprimod (N-N-diethyl-8,8-dipropyl-2-azaspiro [4,5] decane-2-propanamine), a novel cationic amphiphilic compound, has been studied for its anticancer properties in malignancies.9–11 Recent reports have suggested that atiprimod exhibits antiproliferative and antiangiogenic activities, induces apoptosis via activation of caspases-3 and -8, and inhibits phosphorylation of Akt and STAT3 in multiple myeloma (MM).10,12 In addition, atiprimod exhibits in vivo antimyeloma effects in xenograft severe combined immunodeficient (SCID) mouse models.10,13 These results provide a framework for clinical trials of atiprimod in MM.
MCL has distinct biologic, morphologic, and immunophenotypic features in NHL.14 MCL responds to similar agents used effectively for MM. For example, the chemotherapy regimens CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and hyperCVAD (cyclophosphamide, vincristine, doxorubicin, decadron, cytarabine, and methotrexate) are effective in B-cell NHL, especially in MCL,15 but their slight modifications are also effective in MM.16–19 Bortezomib, a potent and specific proteasome inhibitor that was U. S. Food and Drug Administration–approved for relapsed MM, was also proven to be effective in MCL.20 Thalidomide is active in MM, and thalidomide in combination with rituximab induces impressive antitumor activity in relapsed/refractory MCL patients.21 Revlimid, an immunomodulatory drug that produces durable clinical responses in patients with relapsed and refractory MM,22 also is showing activity in clinical trials for treatment of NHL. Thus, we hypothesize that MCL and MM share certain biologic mechanisms in responding to various compound therapies. Atiprimod, which exhibits significant antitumor ability against MM cells by growth inhibition and apoptosis,10 may also prove effective in MCL. In this study, we demonstrate that atiprimod not only inhibited growth and induced apoptosis of MCL cell lines SP53, MINO, Grant 519, Jeko-1, and freshly isolated patient-derived primary MCL cells in vitro, but it was also therapeutic against established human MCL in xenograft SCID mouse models. Of importance, atiprimod exhibited lower cytotoxicity against normal T cells. Furthermore, we attempted to elucidate the mechanism by which atiprimod induced apoptosis of MCL cells. Our results demonstrate that atiprimod activated both caspase-dependent and caspase-independent mitochondrial pathways and induced MCL apoptosis mainly via the caspase-independent, apoptosis-inducing factor (AIF)–mediated pathway. Taken together, these results provide preclinical data for the future use of atiprimod in clinical trials of MCL.
Materials and methods
Reagents
Atiprimod was provided by Callisto Pharmaceuticals (New York, NY) and was solubilized in phosphate-buffered saline (PBS; Mediatech, Herndon, VA) before use. Pan-caspase inhibitor Z-VAD, caspase-8 inhibitor IETD-CHO, and caspase-9 inhibitor Z-LEHD were purchased from Calbiochem (San Diego, CA), dissolved in dimethyl sulfoxide (DMSO), and used at a final concentration of 100 μM. FITC-conjugated annexin V was purchased from Caltag Laboratories (Burlingame, CA). Propidium iodide (PI) and N-phenylmaleimide were purchased from Sigma-Aldrich (St Louis, MO). The human β2-microglobulin (β2M) enzyme-linked immunosorbent assay (ELISA) kit was purchased from Alpha Diagnostic (San Antonio, TX). Fetal bovine serum was purchased from Atlanta Biologicals (Norcross, GA). [methyl-3H] thymidine was purchased from Amersham (Arlington Heights, IL).
Patients' samples
Specimens of spleen, bone marrow aspirates, and peripheral blood were obtained from patients with newly diagnosed MCL. This study was approved by the Institutional Review Board at The University of Texas M. D. Anderson Cancer Center. Mononuclear cells were separated by Ficoll-Hypaque density centrifugation, and MCL cells were isolated using anti-CD20 magnetic microbeads (Miltenyi Biotec, Auburn, CA). Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers. Four MCL cell lines, SP53, Mino, Granta 519, and Jeko-1, were maintained in RPMI-1640 (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum, penicillin (10 000 units/mL; Sigma-Aldrich), streptomycin (10 mg/mL; Sigma-Aldrich), gentamicin (50 mg/mL; Sigma-Aldrich), and l-glutamine (29.2 mg/mL; Life Technologies).
Cell growth assay
The growth inhibitory effects of atiprimod on MCL cell lines, PBMCs, and freshly isolated MCL cells were assessed by 3H-thymidine incorporation assay. Briefly, cells were plated in 96-well plates at a concentration of 5 × 104 cells/well and treated with 0 to 8 μM final concentrations of atiprimod for 48 hours. 3 H-thymidine (1 μCi [0.037 MBq]) was added to each well and incubated for the last 16 hours. All experiments were performed in triplicate. Radioactivity was measured by adding scintillation cocktail and counting on a scintillation beta-counter (PerkinElmer Life and Analytical Sciences, Shelton, CT).
Apoptosis assays
Annexin V–binding assay was used to detect the induction of apoptosis. Cells were seeded in 48-well plates with 2 μM atiprimod for various times, or with different concentrations of atiprimod for 24 hours. To quantify the percentages of cells undergoing apoptosis, annexin V–FITC was used. Briefly, atiprimod-treated cells were washed twice with cold PBS and then resuspended in binding buffer at a concentration of 1 × 106 cells/mL. After incubation, 100 μL of the solution was transferred to a 5-mL tube, to which 5 μL annexin V–FITC and 5 μL PI were added. The tube was gently vortexed and incubated for 15 minutes at room temperature in dark. At the end of the incubation, 300 μL binding buffer was added. Flow cytometric analysis was performed immediately with a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Atiprimod-induced cell apoptosis was also detected by the terminal transferase-mediated dUTP (deoxyuridine triphosphate) nick end labeling (TUNEL) assay using APO-BRDU Kit Direct (BD Biosciences, San Jose, CA). MCL cells were cultured for 48 hours with the addition of different concentrations of atiprimod at 37°C, washed, and fixed with 4% paraformaldehyde in PBS. After washing, cells were permeabilized in 70% ethanol, DNA nick ends were labeled with terminal deoxynucleotidyl transferase (TdT) solution, and analysis was performed using FACScan flow cytometer.
Western blot analysis
MCL cells or normal B cells were cultured with the addition of 2 μM atiprimod in the presence or absence of caspase inhibitors. Cells were harvested, washed twice with cold PBS, and lysed with lysis buffer (Cell Signaling, Danvers, MA). Cell lysates were kept on ice for 30 minutes and centrifuged at 14 000g for 10 minutes at 4°C. For the detection of cytosolic AIF and cytochrome c, we followed the protocol of Cytosol Fractionation Kit (BioVision, Mountain View, CA) to acquire cytosolic fractions. Supernatants were collected and the protein content of each fraction was determined by a Bio-Rad Bradford assay (Bio-Rad, Hercules, CA). Samples were boiled in loading buffer and separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, proteins were transferred onto a nitrocellulose membrane (Bio-Rad), which was incubated with blocking solution (5% nonfat dry milk in PBS containing 0.05% Tween-20) for 2 hours and immunoblotted with anti-PARP (BD Biosciences); caspase-3, Bcl-2, pBcl-2, Bcl-XL, Bad, Bax, cytochrome c, AIF, β-actin (Santa Cruz Biotech, Santa Cruz, CA); caspase-8, caspase-9, STAT3, ERK, Akt, and JNK (Cell Signaling) antibodies. The membrane was finally visualized by incubating the membrane with a chemiluminescence Western blot kit (Pierce Biotechnology, Rockford, IL).
In vivo effects of atiprimod on established MCL
Six- to 8-week-old male CB-17 SCID mice (Harlan, Indianapolis, IN) were housed and monitored in our animal research facility. All experimental procedures and protocols had been approved by the Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center. SCID mice were subcutaneously inoculated in the right flank with 5 × 106 SP53 or Grant 519 cells suspended in 50 μL PBS. Mice were treated with intraperitoneal injections of PBS or atiprimod (25 mg per kg per day) for 6 consecutive days after palpable tumors (≥ 5 mm in diameters) developed. Tumor size and body weight were measured daily, and blood samples were collected weekly. Tumor burdens were evaluated by measuring tumor size and detecting circulating human β2M.
Statistical analysis
All assays were performed in triplicates and expressed as mean values ± SE. Statistical significance of differences observed between experimental groups was determined using the Student t test. P values less than .05 were considered significant.
Results
Atiprimod inhibited the growth of MCL cells in vitro
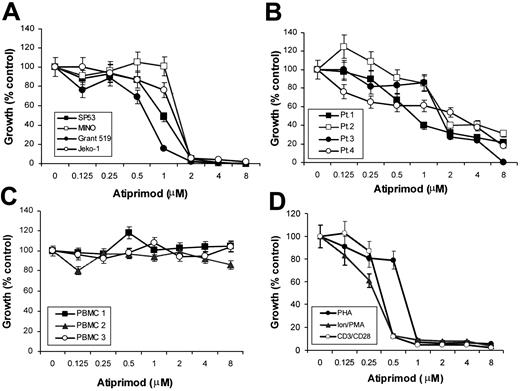
The effects of atiprimod on the growth of 4 MCL cell lines were evaluated using 3H-thymidine incorporation assay. Atiprimod treatment resulted in a significant dose-dependent growth inhibition of SP53, Mino, Grant 519, and Jeko-1 (Figure 1A). Atiprimod demonstrated significant cytotoxicity (> 98% growth inhibition, P < .01), with IC50 (concentration at 50% inhibition) between 1 to 2 μM in the 4 MCL cell lines. The same effects were also observed on patient-derived primary MCL cells. Atiprimod induced growth inhibition of freshly isolated MCL cells from all 4 patients (P < .01) with IC50 between 2 to 4 μM (Figure 1B). We also examined the effects of atiprimod on normal PBMCs from healthy blood donors. Of interest, atiprimod did not affect the proliferation of resting PBMCs (Figure 1C). The presence of atiprimod, however, significantly inhibited the proliferation of activated PBMCs induced by PHA (3 μg/mL), ionomycin (1 μg/mL) and PMA (300 ng/mL), or anti-CD3 mAb (5 μg/mL) and anti-CD28 mAb (1 μg/mL), respectively (Figure 1D). Taken together, these data clearly demonstrate that atiprimod was able to inhibit the proliferation of MCL tumor cells and activated lymphocytes without affecting the growth of normal resting PBMCs.
Effects of atiprimod on the growth of MCL cells and normal PBMCs. (A) Four MCL cell lines: SP53, MINO, Grant 519, and Jeko-1. (B) Freshly isolated primary MCL cells from 4 patients (Pt). (C) Resting PBMCs isolated from 3 healthy volunteers. (D) Activated PBMCs from 3 healthy volunteers by PHA (3 μg/mL), ionomycin (Ion; 1 μg/mL) and PMA (300 ng/mL), or anti-CD3 (5 μg/mL) and anti-CD28 (1 μg/mL) mAbs. Cells were cultured for 48 hours in the presence of 0 to 8 μM atiprimod, with or without the addition of the stimuli. Cell growth was assessed by 3H-thymidine incorporation assay. Results of 3 independent experiments are shown (mean ± SE).
Effects of atiprimod on the growth of MCL cells and normal PBMCs. (A) Four MCL cell lines: SP53, MINO, Grant 519, and Jeko-1. (B) Freshly isolated primary MCL cells from 4 patients (Pt). (C) Resting PBMCs isolated from 3 healthy volunteers. (D) Activated PBMCs from 3 healthy volunteers by PHA (3 μg/mL), ionomycin (Ion; 1 μg/mL) and PMA (300 ng/mL), or anti-CD3 (5 μg/mL) and anti-CD28 (1 μg/mL) mAbs. Cells were cultured for 48 hours in the presence of 0 to 8 μM atiprimod, with or without the addition of the stimuli. Cell growth was assessed by 3H-thymidine incorporation assay. Results of 3 independent experiments are shown (mean ± SE).
Atiprimod induced apoptosis of MCL cells
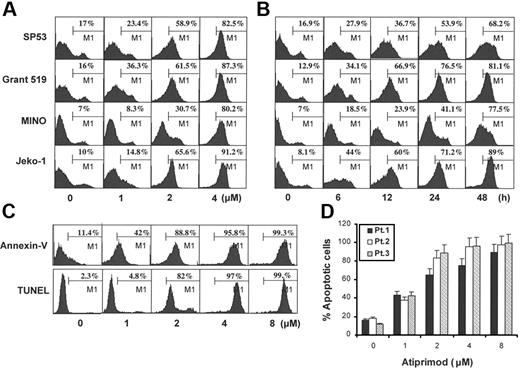
To examine whether apoptosis was responsible for atiprimod-induced growth inhibition, MCL cells were treated with various concentrations of atiprimod for 24 hours, or treated with 2 μM atiprimod for different times. Induction of cell apoptosis was examined by both annexin V–binding and TUNEL assays. Results showed that atiprimod induced apoptosis of all 4 MCL cell lines, SP53, MINO, Grant 519, and Jeko-1, in both a dose-dependent (Figure 2A) and time-dependent (Figure 2B) manner. Atiprimod at concentration greater than 1 μM induced significant apoptosis of the cells as early as 6 hours of culturing (P < .05 to P < .01, compared with medium controls). We also examined freshly isolated, patient-derived primary tumor cells, treated them with different concentrations of atiprimod for 24 hours, and assayed for apoptosis. As shown by the representative histograms of cells from a patient by both annexin V–binding and TUNEL assays (Figure 2C) and data from 3 examined MCL patients (Figure 2D), atiprimod efficiently induced apoptosis of primary MCL cells (P < .05 and P < .01, compared with medium controls) in a dose-dependent manner. Based on these results, we chose to use 2 μM as a standard dose of atiprimod in the following studies with MCL cells.
Atiprimod induces apoptosis of MCL cells. (A) Dose-dependent and (B) time-dependent induction of apoptosis in 4 established MCL cell lines: SP53, MINO, Grant 519, and Jeko-1. (C) Representative histograms of a patient and (D) data from 3 patients showing a dose-dependent induction of apoptosis in freshly isolated primary MCL cells. Cells were treated with different doses of atiprimod for 24 hours and apoptosis was assayed by annexin V–binding and TUNEL assays. Except in Figure 2C, results from TUNEL assay are not shown. Results of 4 independent experiments are shown (mean ± SE).
Atiprimod induces apoptosis of MCL cells. (A) Dose-dependent and (B) time-dependent induction of apoptosis in 4 established MCL cell lines: SP53, MINO, Grant 519, and Jeko-1. (C) Representative histograms of a patient and (D) data from 3 patients showing a dose-dependent induction of apoptosis in freshly isolated primary MCL cells. Cells were treated with different doses of atiprimod for 24 hours and apoptosis was assayed by annexin V–binding and TUNEL assays. Except in Figure 2C, results from TUNEL assay are not shown. Results of 4 independent experiments are shown (mean ± SE).
Cytotoxicity of atiprimod on normal blood cells of healthy volunteers
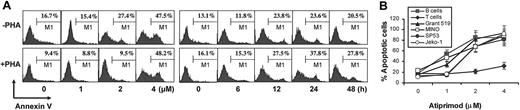
To examine whether atiprimod kills normal PBMCs or lymphocytes, flow cytometry analysis using annexin V–FITC plus CD3-PE or CD19-PE staining was performed. Normal PBMCs were incubated with or without PHA (3 μg/mL) overnight, treated with different concentrations of atiprimod for 24 hours, or treated with 2 μM atiprimod for various times to determine atiprimod-induced cytotoxicity in normal cells. As shown in Figure 3A, atiprimod at 2 μM did not induce apoptosis in normal T cells after a 48-hour incubation with or without PHA stimulation. At 4 μM, atiprimod induced significantly T-cell apoptosis of 24-hour cultures regardless of activation status (P < .05 and P < .01, compared with medium controls). However, B cells were as sensitive as MCL lines to atiprimod (Figure 3B, P < .05 and P < .01, compared with medium controls). These data indicate that atiprimod is also cytotoxic to normal blood cells, especially B cells.
Atiprimod induces apoptosis of normal lymphocytes from healthy volunteers. (A) Representative histograms of cells from one healthy donor showing a dose- and time-dependent induction of apoptosis in resting (−PHA) and PHA (+PHA, 3 μg/mL)–activated, gated CD3+ T cells. (B) Pooled data from 3 healthy donors showing a dose-dependent induction of apoptosis in gated CD3+ T cells and CD19+ B cells in comparison with MCL lines. PBMCs were treated with different concentrations of atiprimod for 24 hours, or treated with 2 μM atiprimod for various times. CD3-PE– or CD19-PE–positive stained cells were gated and annexin V–FITC staining was performed in these gated T cells and B cells, respectively, to determine atiprimod-induced apoptosis in normal lymphocytes. Cell apoptosis was confirmed by TUNEL assay (not shown). Results of 4 independent experiments are shown (mean ± SE).
Atiprimod induces apoptosis of normal lymphocytes from healthy volunteers. (A) Representative histograms of cells from one healthy donor showing a dose- and time-dependent induction of apoptosis in resting (−PHA) and PHA (+PHA, 3 μg/mL)–activated, gated CD3+ T cells. (B) Pooled data from 3 healthy donors showing a dose-dependent induction of apoptosis in gated CD3+ T cells and CD19+ B cells in comparison with MCL lines. PBMCs were treated with different concentrations of atiprimod for 24 hours, or treated with 2 μM atiprimod for various times. CD3-PE– or CD19-PE–positive stained cells were gated and annexin V–FITC staining was performed in these gated T cells and B cells, respectively, to determine atiprimod-induced apoptosis in normal lymphocytes. Cell apoptosis was confirmed by TUNEL assay (not shown). Results of 4 independent experiments are shown (mean ± SE).
Atiprimod triggered apoptosis of MCL cells via the mitochondrial pathways
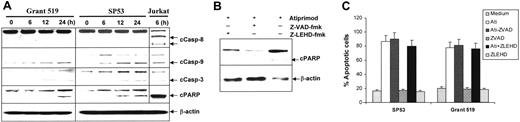
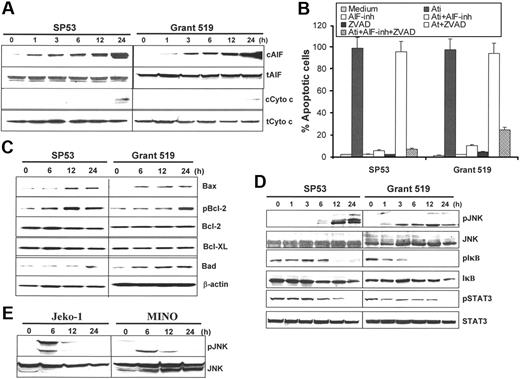
To elucidate the signaling pathways of atiprimod-induced apoptosis in MCL cells, activation and cleavage of caspases and PARP in MCL cell lines treated with or without atiprimod were assessed by Western blot analysis. The results showed that caspase-3 was activated in MCL cells after 6 hours of treatment with atiprimod. Cleavage of PARP was detected after 12 hours of treatment following the activation of caspase-3. Furthermore, we found that atiprimod activated caspase-9, but not caspase-8 (Figure 4A). As a previous study showed that atiprimod activated caspase-8 in treated tumor cells,10 we included a control cell line, Jurkat, which is sensitive to Fas antibody–induced, caspase-8–mediated apoptosis. As expected, anti-Fas mAb (7C11; Immunotech, Miami, FL) activated and cleaved capase-8 and caspase-9 (Figure 4A) and induced apoptosis in Jurkat cells (data not shown). Next, we used caspase inhibitors to investigate the importance of caspase activation in MCL apoptosis. Western blot analysis showed that both the pan-caspase inhibitor Z-VAD and caspase-9 inhibitor Z-LEHD but not caspase-8 inhibitor IETD-CHO (data not shown) abrogated atiprimod-induced PARP cleavage (Figure 4B). However, cell apoptosis, detected by annexin V–binding assay, was not inhibited by pan-caspase or caspase-9 inhibitors (Figure 4C). These results indicate that atiprimod was able to activate caspases-9 and -3, and cleave PARP, which however may not be the main pathway responsible for the induction of apoptosis in MCL cells.
Atiprimod induces caspase activation in MCL cells. (A) Atiprimod-induced activation and cleavage of caspase-9, casapase-3, and PARP. As a control, anti-Fas mAb-induced caspase (-8) activation in Jurkat cells is shown. MCL cells were cultured with 2 μM atiprimod for different times. Jurkat cells were treated with 100 ng/mL anti-Fas mAb for 6 hours. Whole-cell lysates were subjected to Western blot analysis. Pan-caspase inhibitor Z-VAD or caspase-9 inhibitor Z-LEHD completely abrogated the cleavage of PARP (B), but had no effects on atiprimod-induced apoptosis in MCL cells (C). Arrows indicate cleaved (c) forms of caspases and PARP. Ati indicates atiprimod.
Atiprimod induces caspase activation in MCL cells. (A) Atiprimod-induced activation and cleavage of caspase-9, casapase-3, and PARP. As a control, anti-Fas mAb-induced caspase (-8) activation in Jurkat cells is shown. MCL cells were cultured with 2 μM atiprimod for different times. Jurkat cells were treated with 100 ng/mL anti-Fas mAb for 6 hours. Whole-cell lysates were subjected to Western blot analysis. Pan-caspase inhibitor Z-VAD or caspase-9 inhibitor Z-LEHD completely abrogated the cleavage of PARP (B), but had no effects on atiprimod-induced apoptosis in MCL cells (C). Arrows indicate cleaved (c) forms of caspases and PARP. Ati indicates atiprimod.
To further elucidate atiprimod-induced apoptosis in the tumor cells, we examined the involvement and roles of 2 mitochondria-related apoptosis molecules, AIF and cytochrome c. Western blot analysis showed that atiprimod strongly enhanced the release of AIF from mitochondria to cytosol after 12-hour incubation of MCL cells, whereas cytochrome c release into cytosol was not apparent until after 24 hours of culture (Figure 5A). We then used an AIF inhibitor N-phenylmaleimide that inhibits AIF-induced DNA fragmentation,23 and results showed that treatment of MCL cells with N-phenylmaleimide (50 μM) but not Z-VAD completely prevented nucleosomal DNA fragmentation (Figure 5B). These results indicate that, although atiprimod acted on mitochondria to release both cytochrome c and AIF, AIF-mediated, caspase-independent pathway was the main pathway responsible for induction of apoptosis in MCL cells.
Atiprimod-induced intracellular signaling and activation of the mitochondrial apoptotic cascades in MCL cells. Western blot analyses showing the protein levels in cells treated with atiprimod for different times: (A) Cytosolic (c) and total (t) AIF and cytochrome c. (B) MCL cell apoptosis induced by atiprimod in the presence or absence of AIF inhibitor (inh) N-phenylmaleimide and/or pan-caspase inhibitor Z-VAD (ZVAD). Apoptosis was detected by TUNEL assay. (C) Mitochondria-associated proapoptotic and antiapoptotic proteins, including Bax, Bcl-2, and phosphorylated (p) Bcl-2, Bcl-XL, and Bad. (D) JNK and phosphorylated JNK (pJNK), IκB and pIκB, STAT3, and pSTAT3 in MCL SP53 and Grant 519 cells. (E) JNK and pJNK in MCL Jeko-1 and MINO cells. MCL cells were incubated with 2 μM atiprimod for the indicated time points. Whole-cell lysates or cytosolic fractions were collected and subjected to Western blot analysis. Results of 3 independent experiments are shown (mean ± SE).
Atiprimod-induced intracellular signaling and activation of the mitochondrial apoptotic cascades in MCL cells. Western blot analyses showing the protein levels in cells treated with atiprimod for different times: (A) Cytosolic (c) and total (t) AIF and cytochrome c. (B) MCL cell apoptosis induced by atiprimod in the presence or absence of AIF inhibitor (inh) N-phenylmaleimide and/or pan-caspase inhibitor Z-VAD (ZVAD). Apoptosis was detected by TUNEL assay. (C) Mitochondria-associated proapoptotic and antiapoptotic proteins, including Bax, Bcl-2, and phosphorylated (p) Bcl-2, Bcl-XL, and Bad. (D) JNK and phosphorylated JNK (pJNK), IκB and pIκB, STAT3, and pSTAT3 in MCL SP53 and Grant 519 cells. (E) JNK and pJNK in MCL Jeko-1 and MINO cells. MCL cells were incubated with 2 μM atiprimod for the indicated time points. Whole-cell lysates or cytosolic fractions were collected and subjected to Western blot analysis. Results of 3 independent experiments are shown (mean ± SE).
To further confirm the importance of mitochondria in the induction of apoptosis, we examined the involvement of Bcl-2 family, the best-characterized protein family involved in the regulation of apoptotic cell death.23 As shown in Figure 5C, treatment of MCL cells with atiprimod up-regulated the expression of proapoptotic proteins Bax, Bad, and phosphorylated Bcl (pBcl), while the expression of Bcl-XL and Bcl-2 remained unchanged. These results further support our conclusions.
Atiprimod activated JNK and inhibited NF-κB and STAT3
To examine the signaling pathways upstream of the mitochondria, we focused on JNK, NF-κB, and STAT3. Western blot analysis showed that JNK was activated early after 1 hour of incubation of the cells (SP53 and Grant 519) with atiprimod and remained highly activated by 24 hours (Figure 5D). Atiprimod-induced JNK activation in other 2 MCL cell lines Jeko-1 and MINO is shown in Figure 5E. NF-κB is constitutively activated in MCL cells and plays an important role in cell survival and apoptosis.24 SP53 and Grant 519 cells were incubated with atiprimod for different times, up to 24 hours. Western blot analysis showed that atiprimod blocked the phosphorylation of IκB (pIκB), and by 12 hours, phosphorylation of IκB was significantly abrogated (Figure 5D). As inhibited phosphorylation of IκB indicates less degradation of this protein and more bound to NF-κB, these results demonstrate that atiprimod inhibited the activity of NF-κB via blocking the phosphorylation of IκB.
It has been demonstrated that STAT3 is also constitutively activated in a number of human tumors,25–28 including MCL cells,29,30 and possesses tumorigenic potential31 and antiapoptotic activities.25,32 Inhibition of constitutively active STAT3 suppresses proliferation and induces apoptosis.33 In this study, SP53 and Grant 519 cells were incubated with atiprimod for various times. Western blot analysis showed that atiprimod also blocked the phosphorylation of STAT3 in a timely fashion and by 24 hours, phosphorylated STAT3 (pSTAT3) was undetected (Figure 5D). Taken together, these findings indicate that atiprimod treatment resulted in activated JNK and inhibited NF-κB and STAT3 activities in MCL cells.
Atiprimod activated JNK and triggered apoptosis via the mitochondrial pathways in normal B cells
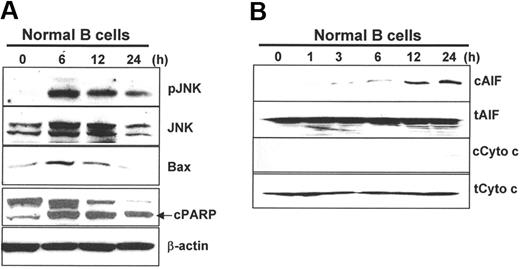
As B cells were sensitive to atiprimod-induced apoptosis, we examined whether the same signaling and apoptosis pathways were activated in the cells. B cells were purified from PBMCs of healthy blood donors using CD19 magnetic beads (Miltenyi Biotec), and some key molecules were examined by Western blot analysis. As shown in Figure 6A, atiprimod activated JNK, up-regulated Bax expression, and activated and cleaved PARP in B cells. Furthermore, atiprimod induced the release of AIF and cytochrome c from mitochondria, although its effect on cytochrome c was much weaker (Figure 6B). Taken together, these results indicate that atiprimod killed MCL and normal B cells via the same mechanisms.
Atiprimod-induced signaling and activation of the mitochondrial apoptotic cascades in normal B cells. Western blot analyses showing the protein levels in B cells treated with atiprimod for different times. (A) JNK and phosphorylated JNK (pJNK), Bax, and cleaved PARP (cPARP). (B) Cytosolic (c) and total (t) AIF and cytochrome c. B cells were incubated with 2 μM atiprimod for the indicated time points. Whole-cell lysates or cytosolic fractions were collected and subjected to Western blot analysis. Results of 3 independent experiments are shown.
Atiprimod-induced signaling and activation of the mitochondrial apoptotic cascades in normal B cells. Western blot analyses showing the protein levels in B cells treated with atiprimod for different times. (A) JNK and phosphorylated JNK (pJNK), Bax, and cleaved PARP (cPARP). (B) Cytosolic (c) and total (t) AIF and cytochrome c. B cells were incubated with 2 μM atiprimod for the indicated time points. Whole-cell lysates or cytosolic fractions were collected and subjected to Western blot analysis. Results of 3 independent experiments are shown.
In vivo effects of atiprimod on established MCL
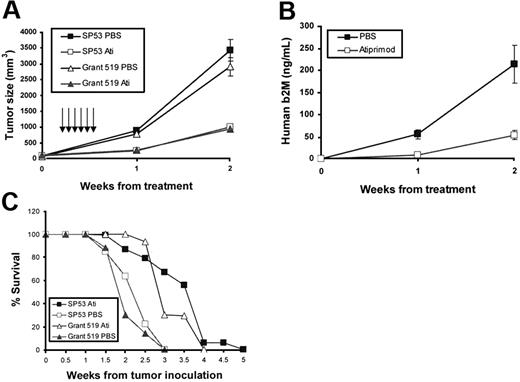
To examine in vivo anti-MCL effects of atiprimod, MCL-SCID mouse models were established. SP53 and Grant 519 cells (5 × 106 cells per mouse) were subcutaneously inoculated into the right flank of SCID mice. When palpable tumors developed (≥ 5 mm in diameter), mice (10 per group) received intraperitoneal injection of atiprimod (25 mg per kg per day) or vehicle control PBS for 6 consecutive days. As shown in Figure 7, mice that received atiprimod treatment had significantly smaller tumor burdens compared with control mice, which was measured by tumor volume (Figure 7A, P < .01 for both cell lines) or level of circulating human β2M (SP53-SCID model only; Figure 7B, P < .05). In addition, atiprimod treatment significantly prolonged the survival of tumor-bearing mice (Figure 7C, P < .05 for both cell lines). Thus, these data clearly demonstrate the in vivo anti-MCL capacity of atiprimod.
In vivo therapeutic effects of atiprimod on established MCL in SCID mouse models. CB-17 SCID mice were inoculated subcutaneously in the right flank with 5 × 106 SP53 cells or Grant 519 cells. Three to 4 weeks later when palpable tumors (≥ 5 mm in diameter) developed, mice (10 per group) were treated with intraperitoneal injections of PBS or atiprimod (25 mg per kg per day) for 6 consecutive days. Tumor burdens were measured as (A) tumor volumes and (B) levels of circulating human β2M in SP53-SCID mouse sera detected by ELISA. (C) Survival of tumor-bearing SCID mice (survival times: atiprimod group versus PBS group: P < .05 in both SP53 and Grant 519 models). Arrows indicate injections of atiprimod or PBS.
In vivo therapeutic effects of atiprimod on established MCL in SCID mouse models. CB-17 SCID mice were inoculated subcutaneously in the right flank with 5 × 106 SP53 cells or Grant 519 cells. Three to 4 weeks later when palpable tumors (≥ 5 mm in diameter) developed, mice (10 per group) were treated with intraperitoneal injections of PBS or atiprimod (25 mg per kg per day) for 6 consecutive days. Tumor burdens were measured as (A) tumor volumes and (B) levels of circulating human β2M in SP53-SCID mouse sera detected by ELISA. (C) Survival of tumor-bearing SCID mice (survival times: atiprimod group versus PBS group: P < .05 in both SP53 and Grant 519 models). Arrows indicate injections of atiprimod or PBS.
Discussion
MCL frequently relapses after therapy; therefore, new therapeutic agents are needed in the clinic. Atiprimod, a cationic amphiphilic compound, has been recently evaluated for its effects on human MM cells and acute myeloid leukemia (AML) cells.10–12 Atiprimod inhibited proliferation and induced apoptosis of these tumor cells via activation of caspase-dependent signal pathway.10–12 In this study, we evaluated the effects of atiprimod on human MCL cells and normal PBMCs. The results demonstrated that atiprimod had high therapeutic efficacy in MCL cells and a low toxicity toward normal cells.
We first demonstrated that atiprimod directly inhibited the growth of 4 MCL cell lines, SP53, MINO, Grant 519, and Jeko-1, in a dose-dependent manner with an IC50 of less than 2 μM. Proliferation of freshly isolated MCL cells from patients was also significantly inhibited. In contrast, the same treatment did not affect the growth of normal resting PBMCs. These results agree with data obtained in myeloma cells by MTT assay.10 Of interest, atiprimod inhibited the proliferation of PHA-, PMA/ionomycin-, or anti-CD3 mAb/anti-CD28 mAb–activated PBMCs, suggesting that atiprimod affects actively dividing/proliferating cells. Using annexin V–binding and TUNEL assays, we found that concentrations of atiprimod higher than 2 μM induced apoptosis in all 4 MCL cell lines and freshly isolated MCL cells from patients after 24 hours of treatment, but it failed to induce apoptosis in normal T cells. The data support the notion that atiprimod is less cytotoxic to normal PBMCs.10 Of importance, the xenograft MCL-SCID mouse model demonstrates that atiprimod significantly inhibited MCL cell growth and prolonged the survival of tumor-bearing mice, indicating that atiprimod is effective and therapeutic to MCL in vivo.
We attempted to elucidate the mechanism of atiprimod-induced apoptosis in MCL cells. First, we found that atiprimod activated and induced cleavage of caspase-3, caspase-9, and PARP. Western blot analysis showed that pretreatment of the cells with caspase-9 or pan-caspase inhibitors, but not caspase-8 inhibitor, completely blocked atiprimod-induced cleavage of PARP. However, pretreatment of the cells with these inhibitors failed to block atiprimod-induced apoptosis. These results suggested that atiprimod-induced apoptosis in MCL cells may not require caspase activation, so we focused on investigating the role of AIF.
AIF is a ubiquitously expressed flavoprotein that plays a critical role in caspase-independent apoptosis.34 AIF is normally localized to the mitochondrial intermembrane space and is released in response to apoptotic stimuli. In this pathway, apoptosis stimuli induce release of AIF from mitochondria to the cytosol and nucleus, with subsequent DNA fragmentation and cell death.35 There are several reports that apoptosis induced via the AIF pathway could also be caspase-dependent because the release of AIF from mitochondria was blocked by the pan-caspase inhibitor Z-VAD.36–39 In our study, the release of AIF protein was caspase independent, since atiprimod-induced AIF release was not blocked by Z-VAD (data not shown). Our study showed that AIF was progressively released to cytosol after 6 hours of incubation of MCL cells with atiprimod. Preincubation of MCL cells with AIF inhibitor N-phenylmaleimide, but not Z-VAD, completely prevented nucleosomal DNA fragmentation. These results indicate that atiprimod triggers apoptosis of MCL cells through a caspase-independent, AIF-mediated pathway.
The mechanisms by which atiprimod induces apoptosis in tumor cells had previously been poorly elucidated and controversial. An early study examining the apoptotic effects of atiprimod on myeloma cells showed that atiprimod induced apoptosis via caspase-8, caspase-3, and PARP.10 The investigators used the pan-caspase inhibitor Z-VAD and showed that it abrogated atiprimod-induced cleavage of PARP. However, no data were provided on whether such a treatment could affect apoptosis of the cells. A subsequent study examining the role of atiprimod on colon carcinoma cells demonstrated that atiprimod induced apoptosis by activating caspase-9 and caspase-3,40 which is different from the study in myeloma. Other studies reported activation of caspase-3 and PARP; however, all10–12,40 failed to examine the role of caspase activation in the induction of apoptosis. In the present study, we showed that atiprimod activates both caspase-9/-3 and AIF pathways, and the latter is responsible for inducing apoptosis in MCL cells. Therefore, it is yet unknown which causes the discrepancy among these studies. A possibility is that atiprimod may act on different pathways in different types of tumor cells.
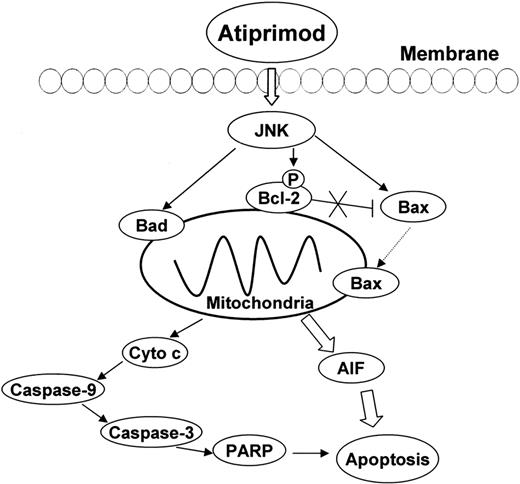
Bcl-2 family members play an important role in the mitochondrial apoptotic pathway. The Bcl-2 subfamily (Bcl-2 and Bcl-XL) functions to inhibit apoptosis, while the Bax subfamily (Bax, Bak, Bok, and Bcl-XS) and the BH3-only subfamily (Bad, Bid, Bik, Bim, Bmf, Noxa, Puma) promote apoptosis.41 To elucidate the upstream proteins in the atiprimod-induced mitochondrial apoptotic pathways, we studied the effect of atiprimod on Bcl-2 family members and their upstream regulator JNK, which mediates signaling to the members of Bcl-2 family.42 The results show that JNK and Bcl-2 were phosphorylated, and the expression of Bax and Bad were progressively up-regulated after treatment of the cells with atiprimod. It has been reported that JNK inactivated the antiapoptotic proteins Bcl-2 by inducing Bcl-2 phosphorylation.43 JNK also transduces apoptotic signaling to Bax, which translocates to mitochondria, and causes permeabilization of the mitochondrial membrane and release of apoptogenic factors such as AIF.44,45 AIF translocates to the nucleus and induces chromatin condensation and large-scale DNA fragmentation. Bad, a proapoptotic Bcl-2 family member protein, can displace Bax bound to Bcl-2 and Bcl-XL, thereby promoting apoptosis.46 Taken together, atiprimod activates JNK by inducing JNK phosphorylation. JNK, as upstream regulator, up-regulates apoptotic proteins Bax and Bad, and inhibits antiapoptotic protein Bcl-2. These Bcl-2 family members, therefore, trigger mitochondrial apoptotic pathways (Figure 8).
Schematic representation of atiprimod-induced signaling and mitochondrial apoptotic pathways in MCL cells. Atiprimod phosphorylates and activates JNK, which in turn up-regulates the expression of proapoptotic proteins Bax and Bad, and phosphorylates and inhibits the activity of antiapoptotic protein Bcl-2 (pBcl-2). Bad displaces Bax from binding to Bcl-2, and JNK transduces apoptotic signaling to Bax, which translocates to the mitochondria, increases the permeability of the mitochondria, and induces the release of AIF and cytochrome c. Based on our results, AIF translocation into nuclei, which induces chromatin condensation and large-scale DNA fragmentation is largely responsible for apoptosis of MCL cells.
Schematic representation of atiprimod-induced signaling and mitochondrial apoptotic pathways in MCL cells. Atiprimod phosphorylates and activates JNK, which in turn up-regulates the expression of proapoptotic proteins Bax and Bad, and phosphorylates and inhibits the activity of antiapoptotic protein Bcl-2 (pBcl-2). Bad displaces Bax from binding to Bcl-2, and JNK transduces apoptotic signaling to Bax, which translocates to the mitochondria, increases the permeability of the mitochondria, and induces the release of AIF and cytochrome c. Based on our results, AIF translocation into nuclei, which induces chromatin condensation and large-scale DNA fragmentation is largely responsible for apoptosis of MCL cells.
NF-κB and STAT3 are constitutively activated in MCL cells.24,47 Our results show that atiprimod inhibited constitutive activation of NF-κB and STAT3, which are involved in the apoptosis, proliferation, and survival of tumor cells.24,33 The activity of NF-κB is tightly regulated by its interaction with inhibitory IκB proteins. NF-κB transcription factors are present in the cytosol in an inactive state, complexed with the IκB.48 Activation occurs via phosphorylation of IκB resulting in the release and nuclear translocation of active NF-κB. Atiprimod inhibited constitutive activation of the NF-κB signaling cascade via completely blocking the phosphorylation of IκB. These effects may contribute to growth inhibition and apoptosis induction in MCL cells.
In conclusion, atiprimod is cytotoxic to both MCL cell lines and freshly isolated MCL cells from patients. Atiprimod inhibited MCL growth and prolonged mouse survival in MCL-SCID mouse models. Atiprimod induced apoptosis via caspase-independent AIF pathway, which was associated with the up-regulation of JNK, Bad, Bax, and pBcl-2. Taken together, our results provide a framework for clinical application of atiprimod in MCL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by institutional start-up funds from The University of Texas M. D. Anderson Cancer Center and funds from the Crutchfield family and the Kimmel family philanthropic foundations.
We thank Alison Woo for providing editorial assistance.
Authorship
Contribution: M.W., L.Z., X.H., and Q.Y. initiated the work, designed the experiments, and wrote the paper; L.Z., X.H., J.Y., J.Q., and S.H. performed the experiments and statistical analyses, and F.S. and J.R. provided patients' samples and critical suggestions to this study. M.W., L.Z., and X.H. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qing Yi, Department of Lymphoma and Myeloma, M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0903, Houston, TX 77030; e-mail: qyi@mdanderson.org.