To the editor:
Allogeneic bone marrow transplantation (BMT) is an established treatment for a variety of hematological and immunological disorders. It is postulated that the beneficial effects after myeloablation and haploidentical BMT are mediated by alloreactive donor natural killer (NK) cells, and host-reactive NK-cell clones have been isolated from human transplant recipients up to 3 months after transplantation.1,2 However, little is known about the ability of graft-derived NK cells to target host immune cells after nonmyeloablation and haploidentical BMT. Likewise, the persistence of donor NK-cell alloreactivity in chimeric hosts is poorly defined in this setting.3 Here, we report the ability of alloreactive donor NK cells to eradicate host immune cells after haploidentical BMT in mice, without inducing graft-versus-host disease (GVHD).
The Experimental Animal Commission of Leiden University Medical Center (Leiden, The Netherlands) approved the experiments described in this letter.
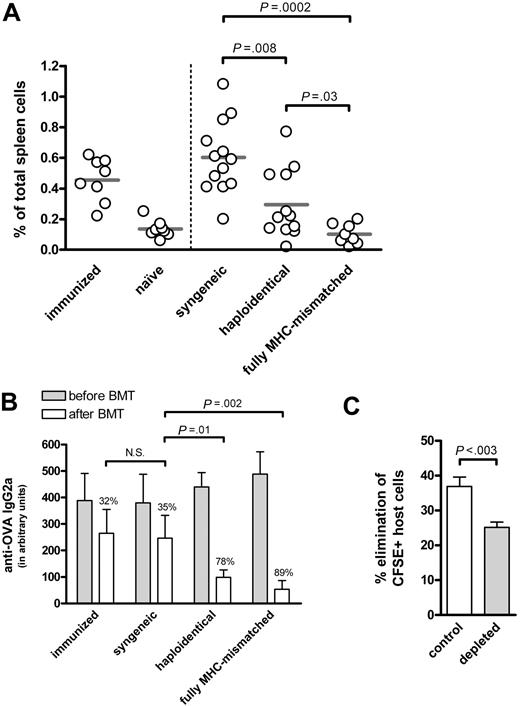
Recently, we showed the effective treatment of B-cell–mediated autoimmune disease (AID) in mice by allogeneic BMT.4 We postulated the rapid eradication of host B cells produced pathogenic autoantibodies by donor NK cells. To further analyze donor NK-cell alloreactivity toward antibody-secreting B cells, we used a model of haploidentical (F1→P) BMT involving host B cells recognizing a model antigen, ovalbumin (OVA). In this model, donor T cells are tolerant for host cells,5,6 but donor NK cells are activated as they lack inhibitory signals provided by self–major histocompatibility complex (MHC) class I.7,8 After immunization, high levels of anti-OVA antibodies were detected. Subsequently, the mice were treated with nonmyeloablative conditioning (ie, low-dose total-body irradiation (6 Gy) and anti-CD40L antibodies (0.5 mg), and BMT from syngeneic, haploidentical or fully MHC-mismatched donor mice. Both fully MHC-mismatched and haploidentical BMT resulted in the early elimination of OVA-specific B cells (Figure 1A) and anti-OVA antibodies (Figure 1B), in contrast to syngeneic BMT. Recipient mice had no signs of acute GVHD (ie, no weight loss and/or detectable histologic abnormalities of liver, gut, and skin), supporting the notion that donor NK cells can mediate beneficial anti–host B-cell alloresponses, without causing GVHD.
Persistent donor NK-cell alloreactivity toward host B cells after haploidentical BMT. (A,B) Haploidentical BMT results in a significant decrease in OVA-specific B cells and anti-OVA antibodies. DBA/1 mice were immunized with OVA, and were either left untreated or were subjected to nonmyeloablative conditioning consisting of low-dose total body irradiation (6 Gy) and a single injection of 0.5 mg of anti-CD40L antibodies (MR1), followed by transplantation with 107 syngeneic, haploidentical (F1→P) or fully MHC-mismatched BM cells from DBA/1, (DBA/1 x C57BL/6) F1 or C57BL/6 donor mice, respectively. The percentage of OVA-specific B cells in spleen was analyzed 2 weeks after BMT. Compared to syngeneic controls, both haploidentical (F1→P) and fully MHC-mismatched BMT recipients showed a significant decrease in the percentage of OVA-specific B cells (P = .008 and P = .0002, respectively). Sera were taken before and after BMT, and tested by ELISA for the presence of anti-OVA IgG2a antibodies. At the start of treatment, no differences in anti-OVA antibody levels were observed between the groups of mice. After treatment, all groups displayed a decrease in anti-OVA antibodies; syngeneic BMT-treated mice showed a decrease in OVA-specific antibody levels of 35%, similar to that of controls (32%), while both haploidentical (F1→P) and fully MHC-mismatched BMT resulted in a strong decrease in antibodies against OVA of 78% (P = .01) and 89% (P = .002), respectively. As negative control, naive mice were analyzed, while immunized mice served as positive control. (N.S. = statistically not significant.) (C) Donor NK cells persistently eradicate host immune cells after haploidentical BMT. At 3 months after BMT, chimeric C57BL/6 mice treated with haploidentical (F1→P) BMT from (BALB/c x C57BL/6) F1 were injected with 107 CFSE-labeled splenocytes (containing 50% to 60% B cells) from syngeneic (CFSEdim) and haploidentical (CFSEhigh) mice mixed at a 1:1 ratio. Before adoptive transfer, NK cells in recipient mice were depleted with 0.25 mg anti-NK1.1 antibodies (PK136), while PBS was used as control. At day +7 after adoptive transfer, the presence of CFSE+ cells in peripheral blood was analyzed by FACS. Error bars indicate mean ± SEM.
Persistent donor NK-cell alloreactivity toward host B cells after haploidentical BMT. (A,B) Haploidentical BMT results in a significant decrease in OVA-specific B cells and anti-OVA antibodies. DBA/1 mice were immunized with OVA, and were either left untreated or were subjected to nonmyeloablative conditioning consisting of low-dose total body irradiation (6 Gy) and a single injection of 0.5 mg of anti-CD40L antibodies (MR1), followed by transplantation with 107 syngeneic, haploidentical (F1→P) or fully MHC-mismatched BM cells from DBA/1, (DBA/1 x C57BL/6) F1 or C57BL/6 donor mice, respectively. The percentage of OVA-specific B cells in spleen was analyzed 2 weeks after BMT. Compared to syngeneic controls, both haploidentical (F1→P) and fully MHC-mismatched BMT recipients showed a significant decrease in the percentage of OVA-specific B cells (P = .008 and P = .0002, respectively). Sera were taken before and after BMT, and tested by ELISA for the presence of anti-OVA IgG2a antibodies. At the start of treatment, no differences in anti-OVA antibody levels were observed between the groups of mice. After treatment, all groups displayed a decrease in anti-OVA antibodies; syngeneic BMT-treated mice showed a decrease in OVA-specific antibody levels of 35%, similar to that of controls (32%), while both haploidentical (F1→P) and fully MHC-mismatched BMT resulted in a strong decrease in antibodies against OVA of 78% (P = .01) and 89% (P = .002), respectively. As negative control, naive mice were analyzed, while immunized mice served as positive control. (N.S. = statistically not significant.) (C) Donor NK cells persistently eradicate host immune cells after haploidentical BMT. At 3 months after BMT, chimeric C57BL/6 mice treated with haploidentical (F1→P) BMT from (BALB/c x C57BL/6) F1 were injected with 107 CFSE-labeled splenocytes (containing 50% to 60% B cells) from syngeneic (CFSEdim) and haploidentical (CFSEhigh) mice mixed at a 1:1 ratio. Before adoptive transfer, NK cells in recipient mice were depleted with 0.25 mg anti-NK1.1 antibodies (PK136), while PBS was used as control. At day +7 after adoptive transfer, the presence of CFSE+ cells in peripheral blood was analyzed by FACS. Error bars indicate mean ± SEM.
Next, we examined whether host-reactive NK-cell alloresponses after haploidentical BMT are still present 3 months after BMT using an in vivo cytotoxicity assay.9,10 In this experiment, differentially carboxyfluoroscein succinimidylester (CFSE)-labeled splenocytes from syngeneic (host) and haploidentical (control) mice were injected into chimeric hosts (4% to 5% host Gr-1+ cells). Prior to the challenge with CFSE-labeled splenocytes, NK cells in recipient mice were (or were not) depleted by anti-NK1.1 antibodies. At day +7, peripheral-blood cells were analyzed for the presence of CFSE+ cells, and the extent of host-cell elimination was determined. Syngeneic cells were eliminated in control mice, while this elimination was inhibited after depletion of NK1.1+ cells (Figure 1C). Although the effect of depletion of NK1.1+ cells is probably underestimated, as not all F1-derived NK-cells express NK1.1, these data indicate that host-reactive NK-cells contribute to the eradication of host immune cells, and that this alloreactivity persists up to 3 months after BMT.
In conclusion, our data suggest that donor NK-cell alloreactivity toward host immune cells may be exploited in the setting of allogeneic BMT to treat AID in which B cells play a pathogenic role.
Authorship
R.F. and G.W. contributed equally to this letter.
Correspondence: Roelof Flierman, Department of Nephrology (D3-P), Leiden University Medical Center, Albinusdreef 2, 2333 ZA, Leiden, The Netherlands, email: r.flierman@lumc.nl
Conflict-of-interest disclosure: The authors declare no competing financial interests.