In this issue of Blood, Antl and colleagues identify inositol-1,4,5-trisphosphate receptor–associated protein (IRAG) as a protein important in the signaling pathway by which nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) mediate platelet inhibition.
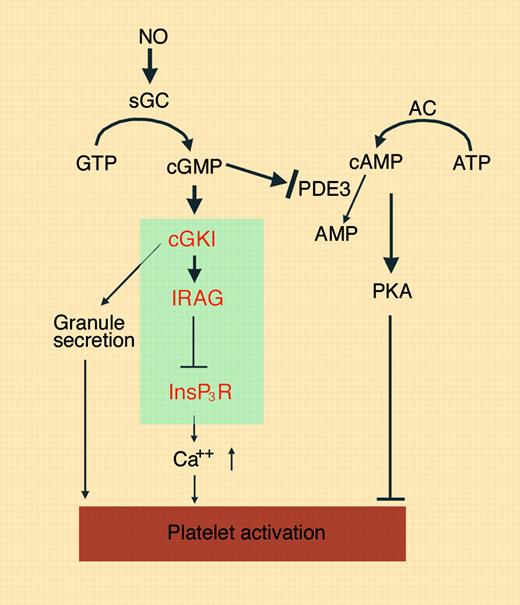
NO and cGMP are reported to play both a stimulatory and an inhibitory role in platelet activation depending on concentration and timing (see figure).1-3 It has been long recognized that the inhibitory effect of NO on platelets is mediated by NO-induced activation of soluble guanylyl cyclase, an enzyme that synthesizes cGMP. cGMP activates the cGMP-dependent protein kinases (cGKs) and also inhibits phosphodiesterase 3, an enzyme that destroys another cyclic nucleotide, cyclic adenosine monophosphate (cAMP). cAMP activates the cAMP-dependent protein kinase (PKA), a known platelet inhibitor. Therefore, NO/cGMP-induced platelet inhibition can be mediated by both cGK- and PKA-dependent mechanisms.4 An important role for cGK type I (cGKI) in the NO/cGMP-mediated platelet inhibition has been convincingly demonstrated by the observation that deletion of the gene encoding cGKI in mice abolishes platelet inhibition caused by preincubation with cGMP analogs.5 However, it has been unclear how cGKI mediates the platelet-inhibitory effects of NO/cGMP. Several proteins have been hypothesized to be cGK substrates/effectors in this regard, including vasodilator-stimulated phosphoprotein (VASP) and the thromboxane A2 receptor, although the evidence so far has not been definitive.
The NO/cGMP-mediated platelet signaling pathways. NO activates cGMP synthesis by sGC. cGMP induces activation of cGKI and inhibition of PDE3, which lead to stimulation or inhibition of platelet activation. The novel cGKI-dependent platelet inhibition mechanism described in this issue of Blood involves cGK phosphorylation of IRAG, which negatively regulates InsP3 receptor (InsP3R)–dependent calcium elevation, and thus causes inhibition of platelet activation. Illustration by Marie Dauenheimer.
The NO/cGMP-mediated platelet signaling pathways. NO activates cGMP synthesis by sGC. cGMP induces activation of cGKI and inhibition of PDE3, which lead to stimulation or inhibition of platelet activation. The novel cGKI-dependent platelet inhibition mechanism described in this issue of Blood involves cGK phosphorylation of IRAG, which negatively regulates InsP3 receptor (InsP3R)–dependent calcium elevation, and thus causes inhibition of platelet activation. Illustration by Marie Dauenheimer.
NO donor compounds or cGMP analogs have been shown to inhibit platelet agonist–induced elevation of intracellular Ca2+, which is a critical event in platelet activation. In smooth muscle cells, a cGKI substrate protein, IRAG, is associated with both cGKI and the inositol-1,4,5-trisphosphate (InsP3) receptor. The latter is important in the release of calcium from intracellular stores and thus calcium elevation. IRAG indeed negatively regulates InsP3-induced calcium release and smooth muscle relaxation. In the present study, Antl and colleagues found that mouse platelets expressing a deletion mutant of IRAG lacking the N-terminal 12 amino acid residues involved in InsP3 receptor interaction diminished the inhibitory effects of NO donor compounds and cGMP analogs on platelet aggregation, integrin activation, and calcium elevation. Consistent with these in vitro effects, this IRAG mutation also abolished the inhibitory effects of NO donors and cGMP analogs on the incorporation of infused platelets into thrombi in vivo in the mouse. An interesting twist to the story is that the IRAG deletion mutation did not significantly affect platelet function and in vivo thrombosis in the absence of exogenous NO donors and cGMP analogs, which may be caused by either an insufficient sensitivity of the experimental conditions or a difference between endogenous platelet NO/cGMP and exogenously added NO/cGMP in regulating platelet function. Overall, as discussed by the authors, while alternative explanations are possible, the results presented in this paper provide the most convincing evidence thus far for a downstream signaling mechanism that mediates the cGK-dependent inhibition of platelet function by NO and cGMP (see figure).
Conflict-of-interest disclosure: The authors declare no competing financial interests. ▪