Abstract
In immunoglobulin light chain (AL) amyloidosis, amyloid fibril deposits derived from immunoglobulin light chains produced by a clonal plasma cell dyscrasia accumulate in tissues and damage vital organs. Treatment regimens used in multiple myeloma can be effective in AL amyloidosis; however, patients with this disease often tolerate these regimens poorly because of multisystem organ dysfunction. Thalidomide and lenalidomide have both been shown to be effective in myeloma. In this report, we describe results of a phase 2 trial of the use of lenalidomide, as a single agent and in combination with dexamethasone, for the treatment of AL amyloidosis. Thirty-four patients with AL amyloidosis, most with prior therapies, were enrolled in the trial. The initial dose of lenalidomide used (25 mg/d) was poorly tolerated; however, a reduced dose of 15 mg/d was generally well tolerated. Of 24 evaluable patients, 7 (29%) achieved a hematologic complete response and 9 (38%) achieved a partial hematologic response, for an overall hematologic response rate of 67%. Hematologic responses were also associated with clinical responses. Fatigue and myelosuppression were the most common treatment-related adverse events (35%), while thromboembolic complications (9%) were the most serious. Findings from this trial indicate that lenalidomide can be effective in treating AL amyloidosis.
Introduction
Immunoglobulin light chain (AL) amyloidosis is the most common form of systemic amyloidosis. In this lethal disorder, there is widespread deposition of amyloid fibrils derived from the monoclonal immunoglobulin light chain associated with an underlying plasma cell dyscrasia. Amyloid deposits progressively damage various organs and tissues. The median survival of untreated patients is 13 months from diagnosis and is increased marginally to 16 to 18 months when patients are treated with oral low-dose cyclic melphalan and prednisone.1,2 However, this form of treatment rarely results in eradication of the underlying plasma cell dyscrasia and reversal of organ dysfunction. In contrast, high-dose intravenous melphalan and autologous peripheral blood stem cell transplantation (HDM/SCT) can induce hematologic and clinical remissions and prolong survival substantially.3 However, HDM/SCT is suitable for only a select subset of patients with AL amyloidosis. In a large series of patients seen at our institution, only 56% were deemed eligible for HDM/SCT.3 Moreover, while durable remissions were achieved in 40% of the patients treated by HDM/SCT, significant treatment-related morbidity and mortality were observed. Therefore, alternative effective therapies are clearly needed both for patients ineligible for HDM/SCT and for those with persistent or progressive disease following such treatment.
The immunomodulatory agents thalidomide (Thalomid) and lenalidomide (Revlimid, CC-5013) have both been shown to have significant therapeutic activity in the treatment of newly diagnosed4,5 and relapsed/refractory multiple myeloma.6 Based on observations with myeloma treatment regimens that include thalidomide, this drug has been studied as a single agent7,8 and in combination with dexamethasone7-9 in the treatment of AL amyloidosis. When combined with dexamethasone, response rates of nearly 50% were observed.9 Unfortunately, this regimen was found to be very poorly tolerated; 50% to 65% of patients experienced grade 3 and 4 toxicities with treatment, necessitating discontinuation of therapy in up to half of the patients treated with this drug.
Lenalidomide is an analog of thalidomide that has significantly more therapeutic activity in preclinical myeloma models than thalidomide.10 It has also been shown to be effective both alone and in combination with dexamethasone in the treatment of relapsed and refractory myeloma patients as well as patients with newly diagnosed myeloma. In addition, treatment responses have been observed in patients who had previously failed thalidomide, and it appears to have fewer nonhematologic side effects than thalidomide.5,10 These considerations prompted us to design a prospective phase 2 clinical trial of lenalidomide in the treatment of AL amyloidosis. The objectives of this trial were to determine the feasibility and tolerability of lenalidomide treatment in AL amyloidosis, both by itself and in combination with dexamethasone, as well as to determine whether hematologic and clinical responses occurred.
Patients, materials, and methods
Eligibility criteria
The clinical trial was approved by the Institutional Review Board of the Boston University Medical Center in accordance with federal regulations and the Declaration of Helsinki. Eligibility for this study, begun in 2004, required a tissue diagnosis of amyloidosis with evidence of an underlying clonal plasma cell dyscrasia (as indicated by an abnormal clonal dominance of plasma cells in the bone marrow and/or detection of a monoclonal gammopathy by immunofixation electrophoresis of serum and urine proteins and/or an abnormal serum free light chain ratio). Patients were required to be at least 18 years of age. Previous treatment for AL amyloidosis was allowed but had to have been discontinued more than 4 weeks prior to enrollment on the trial. Other eligibility criteria included a blood hemoglobin measurement above 80 g/L (8 g/dL), platelet count above 100 × 109/L, white blood cell count above 3.0 × 109/L, ANC above 1.0 × 109/L, AST/ALT less than twice the upper limit of normal (ULN), as well as a measure of performance status by Southwest Oncology Group (SWOG) of 2 or below. Patients in end-stage renal failure and on dialysis were not eligible.
Pregnant or nursing women, as well as women of childbearing potential who were unwilling to use a dual method of contraception, and men who were unwilling to use a condom were not eligible for the study. Women of childbearing age were required to have a pregnancy test every 4 weeks if their periods were regular and every 2 weeks if their periods were irregular.
Treatment protocol
Lenalidomide was initiated at 25 mg/d for 21 days of a 28-day cycle. If there was no evidence of a hematologic response after 3 cycles of single-agent lenalidomide, pulse dexamethasone was added at 10 to 20 mg/d from days 1 to 4, 9 to 12, and 17 to 20 every other cycle. The dose of dexamethasone was determined by the treating physician based upon age and upon prior tolerance of dexamethasone. A proton pump inhibitor was given to patients while receiving dexamethasone. Also, as described in “Treatment-related toxicities,” daily oral aspirin prophylaxis was added to the treatment regimen during the course of the trial.
Protocol treatment was continued until disease progression or the development of unacceptable toxicities occurred.
Dose modifications
Dose adjustments were permitted based on toxicity. Lenalidomide was discontinued if any of the following toxicities occurred: a desquamating rash or other rash of grade 4 severity, grade 4 neuropathy or hypersensitivity, or at least a grade 3 bradycardia or other cardiac arrhythmia. Subjects who experienced other grade 3 or 4 toxicities potentially related to lenalidomide discontinued treatment until resolution of the adverse event; treatment was then restarted at the next lower dose level. If an isolated neutropenia occurred, addition of granulocyte colony-stimulating factor (G-CSF) treatment was permitted in lieu of lenalidomide dose reduction. Lenalidomide dose reductions were initially from 25 mg to 15 mg and then to 10 mg and 5 mg. When grade 3 or 4 adverse events occurred prior to day 15 of a cycle but resolved to grade 2 or below prior to day 21 of the cycle, lenalidomide was resumed at the next lower dose level until the cycle was completed, and this reduced dose level was continued with the next cycle. When grade 3 or 4 adverse events occurred on or after day 15 of a given cycle, lenalidomide was held for the remainder of the cycle and reduced by one dose level beginning with the next cycle.
Dose reductions were also permitted for dexamethasone-related toxicities, with a lowering of the dose of dexamethasone initially to 10 mg/d on days 1 to 4, 9 to 12, and 17 to 20, then to 5 mg/d on days 1 to 4, 9 to 12, and 17 to 20, and finally to 5 mg/d on days 1 to 4 and 15 to 18. Therapy was discontinued if patients were unable to tolerate the lowest doses of lenalidomide and/or dexamethasone.
Toxicity and response criteria
The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3, was used to grade adverse events as well as to assign perceived attribution of these events to the study treatment regimen. By these criteria, toxicity was defined as an adverse event considered to be possibly, probably, or definitely related to treatment.
The primary end point of this trial was response rate estimated based on the best response to therapy for each patient during the course of treatment. The response criteria for hematologic and clinical/organ response used were standards defined by the consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis.11 A hematologic complete response12 (CR) was defined as absence of monoclonal protein in serum and urine by immunofixation electrophoresis, a normal bone marrow biopsy with less than 5% plasma cells and absence of κ or λ clonal predominance by immunohistochemistry, and a normal immunoglobulin free light chain ratio. A hematologic partial response (PR) was defined as a 50% or more improvement in quantifiable measures of plasma cell dyscrasia (ie, free light chain concentration and ratio, monoclonal [M] spike, or marrow plasmacytosis).
Statistical design and analysis
The end points of this trial were the safety and efficacy of lenalidomide as single agent or in combination with dexamethasone. All patients meeting the eligibility criteria who had signed a consent form and completed at least 3 cycles were evaluated for response. Twenty-five patients with AL amyloidosis were to be accrued. This was subsequently increased to 35 patients to allow for 25 evaluable patients.
A 2-stage design was implemented with early stopping if the treatment failed to meet a lower efficacy threshold in the first 15 patients.13 A response probability of 25% or greater was of interest, while further testing was not to be pursued if the response probability was 5% or lower. Initially 15 patients were to be accrued. If at least 1 response was observed, 10 additional patients were to be accrued. Four or more responses out of 25 were considered to be evidence warranting further study, provided other factors such as toxicity and survival also appeared favorable. The design had a significance level (probability of falsely declaring an agent with a 5% response probability to warrant further study) of 5% and a power (probability of correctly declaring an agent with 25% response probability to warrant further study) of 90%.
Results
Patient characteristics
From March 2004 to May 2006, 34 patients with AL amyloidosis were enrolled on the trial. The median age of enrolled patients was 65 years (range, 44 to 84 years). There were 24 men and 10 women enrolled. Among enrolled patients, 24 had a λ and 10 a κ clonal plasma cell dyscrasia. The distribution of organ involvement was typical for AL amyloidosis. Thirteen patients had isolated renal involvement, 13 had cardiac involvement, and 7 had more than 2 organ systems involved. Six patients at the time of enrollment had abnormal renal function, with a serum creatinine level of at least 176.8 μmol/L (2.0 mg/dL), and 17 patients had elevated levels of brain natriuretic peptide (BNP) above 100 pg/mL. The median concentration of BNP for all patients was 121 pg/mL (range, 9 to 1353 pg/mL). Thirty-one of the patients (91%) had received prior treatment for AL amyloidosis, and 19 (56%) had received 1 or 2 cycles of HDM/SCT. The median time following HDM/SCT up to the time of enrollment on the trial was 36 months (range, 9 to 78 months). The median number of prior treatment regimens was 1 (range, 0 to 5), and 7 patients had undergone prior treatment with thalidomide (Table 1).
Treatment experience
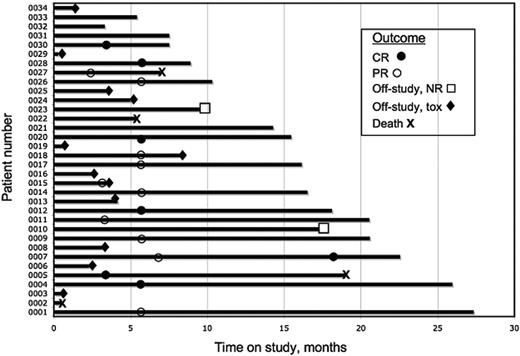
The treatment course and outcomes for the study patients are summarized schematically in Figure 1. Of the 34 patients enrolled, 24 patients completed at least 3 cycles of therapy, and the median number of cycles completed by all the patients has been 6 to date (range, 0 to 23). Ten of the 34 patients were not evaluable for treatment response at the time of data analysis for this report, either because they had been withdrawn from the study (n = 9) or had not completed at least 3 cycles of treatment (n = 1).
Outcome analysis. Each bar represents an individual patient. The abscissa represents the time on study. Significant clinical outcomes for each patient are indicated with symbols for hematologic CR (•), hematologic PR (○), removal from study because of hematologic nonresponse (NR, □) or toxicity (tox, ♦), or death (✖w).
Outcome analysis. Each bar represents an individual patient. The abscissa represents the time on study. Significant clinical outcomes for each patient are indicated with symbols for hematologic CR (•), hematologic PR (○), removal from study because of hematologic nonresponse (NR, □) or toxicity (tox, ♦), or death (✖w).
Six of the 9 patients withdrew because of toxicity (skin rash, n = 2; fatigue, n = 2; pancreatitis, n = 1; worsening renal function, n = 1), 1 patient due to choice, and 2 patients due to early disease progression.
The first 8 patients, treated with lenalidomide at a dose of 25 mg/d, required dose reductions within the first 2 cycles of treatment. Therefore, the protocol was amended such that subsequent patients began treatment at a lower dose (15 mg/d). While this lower dose was better tolerated, most patients eventually required additional dose reductions because of toxicities. Six patients continue or have completed therapy at 15 mg/d, 10 patients continue or have completed therapy at 10 mg/d, and 10 patients continue or have completed therapy at 5 mg/d.
Of the 34 patients enrolled, 16 patients continue to be treated on the study, while 18 (53%) have discontinued treatment because of death (n = 4, 12%), patient choice (n = 1, 3%), absence of hematologic response (n = 2, 6%), or toxicities (n = 11, 32%).
Deaths
Four patients on study have died; however, each of these deaths has been associated with clinically evident amyloid disease progression. Two deaths were caused by sudden cardiac arrhythmias and arrest; another patient died with progressive disease associated with severe diarrhea and dehydration and was off treatment for more than 3 weeks; and the fourth death occurred during acute rejection of a cardiac allograft while on lenalidomide.
Treatment-related toxicities
Treatment-related toxicities and adverse events are summarized inTable 2 Grades 3 and 4 myelosuppression occurred in 9 patients (35%) but generally improved with reduction of the lenalidomide dose. Grades 3 and 4 neutropenia occurred in 6 (18%) and 2 (6%) patients, respectively, while grade 3 thrombocytopenia occurred in 1 patient (3%) and grade 3 anemia in 2 (6%). One patient also developed grade 3 leukopenia. G-CSF treatment permitted 1 patient who developed neutropenia to continue on study. Severe fatigue that was dose related occurred in 9 patients (35%).
Skin rash occurred in 20 patients (59%); this usually abated after treatment was withheld and antihistamines (and in some cases short courses of prednisone) were given. In 6 patients (18%), the rash was extensive, and 2 patients were removed from study because a desquamating rash persisted.
Twenty patients (59%) had worsening serum creatinine and renal function, of which 19 had pre-existing amyloid nephropathy, and 4 had a serum creatinine level of 176.8 μM (2.0 mg/dL) at the time of enrollment. Thirteen patients developed a creatinine above 176.8 μM (2.0 mg/dL) on study, and 2 patients required dialysis for end-stage renal failure. In 10 patients (29%), hypoalbuminemia worsened significantly during treatment.
Respiratory infections occurred in 17 (50%) of the study patients, and 4 (12%) developed radiographically evident pneumonias. None of these infections occurred in association with neutropenia, and they occurred at all dose levels of lenalidomide. Of the 17 patients who developed respiratory infections, 8 were receiving dexamethasone.
Deep venous thrombosis occurred in 2 patients, 1 with lenalidomide alone and 1 with lenalidomide and dexamethasone. A third patient suffered a cerebrovascular accident during air travel while on lenalidomide alone. Of these 3 patients (9%) with thromboembolic complications, 2 had been receiving erythropoietin injections. Notably, 8 other patients received erythropoietic growth factors on the study but did not develop thromboembolic complications. Because of these adverse events and reports of thromboembolic complications in myeloma patients on lenalidomide,12,14 aspirin prophylaxis (81 or 325 mg/d at physician discretion) was prescribed to all subsequent patients while on study. No thromboembolic complications have been observed since the addition of aspirin prophylaxis.
Hematologic responses
Hematologic responses are summarized inTable 3 Hematologic complete responses (CRs) occurred in 2 patients treated with lenalidomide alone and in 5 patients with lenalidomide plus dexamethasone. Thus, 29% (7 of 24) of patients who completed at least 3 cycles of treatment achieved a hematologic CR on this study. In addition, 38% (9 of 24) of patients achieved a hematologic partial response (PR), either with lenalidomide alone or with a combination of lenalidomide and dexamethasone. Therefore, the overall hematologic response rate was 67% (16 of 24), and all of these responses were observed in patients with AL amyloidosis who had had persistent disease despite prior treatment. The response rate by intention to treat was 47% (16 of 34). The median time to hematologic response was 6 cycles (range, 3 to 6), and hematologic responses were observed at all dose levels of lenalidomide.
While the overall durability of hematologic responses is not yet known, 2 patients continue to be in hematologic CR 6 months after discontinuing treatment. Other patients with hematologic responses are either continuing to be treated on the study protocol or have not yet been reevaluated after discontinuing treatment.
Clinical responses
Clinical responses were also evident on this trial. Forty-one percent (6 of 17) of the patients with renal involvement experienced a more than 50% reduction in urinary protein excretion without worsening of renal function. One patient had clinical improvement in symptoms of congestive heart failure as well as a decrease in serum BNP (from 1017 pg/mL to 600 pg/mL) and in diuretic requirement.
Discussion
In a previous trial, we found thalidomide to be very poorly tolerated by patients with AL amyloidosis, because of severe fatigue, constipation, neuropathy, and exacerbation of cardiac and renal dysfunction.7 In contrast, we have found lenalidomide, with dose modifications and appropriate supportive care, to be much better tolerated. While treatment-related fatigue occurred frequently, it was generally less debilitating than that observed with thalidomide, and it was minimized with dose reduction. Myelosuppression was frequently observed in this heavily pretreated patient population and variably included leukopenia, anemia, and thombocytopenia; however, cytopenias were not profound, and no episodes of neutropenic fever occurred. One patient was maintained on study with the use of erythropoietin and G-CSF. At the same time, lenalidomide appeared to have immunosuppressive effects independent of myelosuppression, because half the patients on study developed respiratory infections. Therefore, antibiotic prophylaxis may be an appropriate adjunct to treatment in patients likely to be susceptible to infection.
Unlike thalidomide, lenalidomide did not appear to cause constipation or peripheral neuropathy. However, treatment-related skin rashes were common and occasionally extensive. Nonetheless, in most cases, treatment could be continued following dose reductions and the addition of antihistamines and, in some cases, short, tapering courses of prednisone. Moreover, rashes did not worsen with repeated cycles of treatment.
The most life-threatening complication of lenalidomide therapy was thromboembolism. Three patients experienced thromboembolic events, 2 on lenalidomide alone and 1 on the combination of lenalidomide and dexamethasone. At the time of the adverse event, 2 patients were receiving erythropoietin, which has been reported to increase thromboembolic risk in patients with multiple myeloma while receiving lenalidomide.14 However, no thromboembolic adverse events were seen after aspirin prophylaxis was added to the treatment regimen, suggesting that aspirin is effective in preventing this complication.
Lenalidomide, alone and in combination with dexamethasone, induced hematologic responses in 67% of patients with AL amyloidosis who received at least 3 cycles of treatment. Only 2 patients (8%) experienced progression of hematologic measures of disease while on protocol, and 4 (17%) experienced progression of their clinical amyloid syndrome. This response rate is noteworthy given that almost all patients enrolled on this trial had failed prior treatment, more than half had undergone HDM/SCT, and 7 patients had previously received thalidomide. Five of these 7 achieved a hematologic response with the combination of lenalidomide and dexamethasone (4 PR and 1 CR). The hematologic CR rate was 29%. While these response rates are not as high as those observed with HDM/SCT (42%3 ), they are comparable to the response rates reported for the combination of oral melphalan and dexamethasone.15 Thus, lenalidomide appears to represent an effective treatment alternative to melphalan-based regimens. Furthermore, the hematologic responses produced by this regimen were associated with improvement in organ dysfunction caused by amyloid disease. However, further follow-up and larger trials will be required to determine if these treatment-related responses are associated with improved survival.
Presented in abstract form at the 42nd annual meeting of the American Society of Clinical Oncology, Atlanta, GA, June 5, 2006.16
An InsideBlood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: J.B.Z. is employed by Celgene Corp, whose product (lenalidomide) was studied in the present work. The remaining authors declare no financial conflicts.
Contribution: V.S. designed research, performed research, analyzed data, and wrote the manuscript; D.G.W. edited the manuscript; M.R. performed research and edited the manuscript; K.T.F. collected and analyzed data and designed research; S.F. collected and analyzed data; J.B.Z. designed research and edited the manuscript; M.S. edited the manuscript with critical review; and D.C.S. designed research, performed research, analyzed data, and critically reviewed the manuscript.
Acknowledgments
We gratefully acknowledge the numerous colleagues in the Amyloid Treatment and Research Program, Clinical Trials Office, and Center for Cancer and Blood Disorders at Boston University Medical Center who assisted with the multidisciplinary evaluation and treatment of the patients with AL amyloidosis.
This work was supported by grants from the National Institutes of Health (P01 HL68705), the Gerry Foundation, the Amyloid Research Fund at Boston University, and Celgene (Summit, NJ).