Abstract
Vaccinia virus (VV) has been used extensively as a vaccine vehicle in the clinical application for infectious diseases and cancer. Previous studies have suggested that the unique potency of VV-based vaccine lies in its effective activation of the innate immune system. However, how VV activates innate immune pathways remains largely unknown. In this study, we showed that VV elicited innate immune response through both Toll-like receptor (TLR)–dependent and –independent pathways. The TLR pathway was mediated by TLR2 and MyD88, leading to the production of proinflammatory cytokines, whereas activation of the TLR-independent pathway resulted in the secretion of IFN-β. More importantly, both TLR-dependent and -independent pathways were required for activating innate and adaptive immunity to VV in vivo. These findings represent the first evidence that innate immune recognition of VV is mediated by TLR2, demonstrate that one pathogen can target both TLR and non-TLR innate immune pathways to work together in achieving efficient activation of host defense, and suggest potential new strategies for the design of effective vaccines.
Introduction
Vaccinia virus (VV) belongs to a member of the Orthopoxvirus genus of the Poxviridae family, which includes smallpox (variola) virus, monkeypox virus, cowpox virus, and ectromelia virus.1 It is an enveloped, double-stranded DNA virus with a genome measuring about 200 kb that encodes most of the proteins required for cytoplasmic replication of the virus in host cells. VV is the most studied member of the poxvirus family and is responsible for the successful elimination of smallpox worldwide.2 This success has led to the development of recombinant VV as a vaccine vehicle for other infectious diseases such as influenza, HIV, and malaria.3-5 Furthermore, VV has also been developed as a vaccine for cancer immunotherapy.6 In most cases, immunization with recombinant vaccinia encoding a tumor antigen generates significantly greater immune responses than immunization with the corresponding protein or peptide epitopes mixed with standard adjuvants. So far, many poxviruses have been explored as tumor vaccine vectors and used in human clinical trials, including attenuated, replication-deficient poxviruses such as modified vaccinia Ankara, fowlpox, and canarypox.7-10
What then is responsible for the unique potency of VV as a vaccine vehicle? Using a model of peripheral T-cell tolerance, which is analogous to clinically established cancer, we have recently shown that sustained stimulation of Toll-like receptors (TLRs) of the innate immunity is required for breaking established regulatory T-cell (TReg)–mediated CD8 T-cell tolerance in vivo and that recombinant VV vaccine can provide TLR signals, leading to the reversal of TReg-mediated tolerance, and protect mice from tumor challenge.11 These results suggest that effective activation of the innate immune system contributes to the unique potency of VV vaccine. However, how VV activates innate immune pathways remains unknown. A clear understanding of the mechanisms underlying innate immune response to VV will help us design effective vaccines.
The phylogenetically conserved innate immune system represents the first line of defense against invading pathogens through recognition of pathogen-associated molecular patterns (PAMPs) by a set of receptors called pattern recognition receptors (PRRs).12 TLRs are the best-characterized family of PRRs and are expressed mainly on macrophages and dendritic cells (DCs). Each of 12 TLRs identified in mammals so far appears to recognize a unique set of PAMPs, including peptidoglycan (PGN; TLR2 ligand), dsRNA (TLR3 ligand), lipopolysaccharide (LPS; TLR4 ligand), and CpG DNA (TLR9 ligand).12 Recogition of TLRs by PAMPs triggers signaling cascades leading to the induction of antimicrobial genes and inflammatory cytokines. In addition, emerging evidence supports the existence of TLR-independent innate immune recognition of pathogens.13
In this study, we provided evidence that VV activated innate immunity through both TLR-dependent and -independent pathways, leading to the production of proinflammatory cytokines and interferon-β (IFN-β) in vitro and in vivo. The secretion of proinflammatory cytokines was mediated by TLR2 and dependent on the common TLR adaptor MyD88, whereas the production of IFN-β was through a TLR-independent pathway. Furthermore, we demonstrated that the TLR2/MyD88 innate immune pathway played an important role in shaping innate and adaptive immune responses to VV in vivo. In addition, both innate and adaptive immunity against VV infection in vivo was critically dependent on TLR-independent production of IFN-β. These results indicated that innate immune recognition of VV is mediated by TLR2 and showed that one pathogen can target both TLR and non-TLR innate immune pathways cooperatively to achieve efficient activation of the host's immune system.
Materials and methods
Mice
C57BL/6 mice (H-2b) were purchased from The Jackson Laboratory (Bar Harbor, ME). TLR2−/−,14 TLR9−/−,15 MyD88−/−,16 and TRIF−/−17 mice on C57BL/6 background were kindly provided by Shizuo Akira at Osaka University, Osaka, Japan. TLR4-deficient C57BL/10ScNJ (H-2b) and their normal control mice C57BL/10ScSnJ were also obtained from The Jackson Laboratory as described.18 IFN-αβR−/− mice19 on 129/Sv background (H-2b) and their normal control 129/Sv mice were purchased from B&K Universal (Grimston, United Kingdom). Groups of 6- to 8-week-old mice were selected for this study. All experiments involving the use of mice were done in accordance with protocols approved by the Animal Care and Use Committee of Duke University.
Vaccinia virus
The Western Reserve (WR) strain of VV was purchased from American Type Culture Collection (Manassas, VA). The virus was grown in TK-143B cells and purified by centrifugation through a 35% sucrose cushion as described.11 The titer of virus was determined by plaque assay on TK-143B cells and stored at −80°C until use. UV-inactivation of VV was performed as described,20 with some modifications. Briefly, purified virus was resuspended in 1 μg/mL 8-methoxypsoralen (Sigma, St Louis, MO) and then exposed to a 365-nm UV light source (UVP model UVGL-25) on ice for 3 minutes in a 24-well plate. For in vitro DC stimulations, both live VV and UV-inactivated VV were used at a multiplicity of infection (MOI) of 1. The dose of UV-VV was based on preinactivation titer. For in vivo studies, 1 × 107 pfu of live VV in 0.1 mL phosphate-buffered saline (PBS) was injected into mice intravenously or intraperitoneally as described.11
DC culture
DCs were generated as described.11 Briefly, femurs and tibiae of mice were harvested and bone marrow cells were flushed out with DC medium (RPMI-1640 with 5% fetal bovine serum [FBS], 2 mM l-glutamine, 10 mM HEPES, 50 μM β-mercaptoethanol, 100 IU/mL penicillin, and 100 IU/mL streptomycin). After lysis of red blood cells, the bone marrow cells were cultured in 6-well plates at a density of 3 × 106/mL in 3 mL DC medium in the presence of mouse granulocyte macrophage–colony-stimulating factor (GM-CSF; 1000 U/mL) and interleukin 4 (IL-4; 500 U/mL; R&D Systems, Minneapolis, MN). GM-CSF and IL-4 were replenished on days 2 and 4. On day 5, DCs were harvested, and the same numbers of CD11c+ DCs at a density of 0.85 × 106/mL were transferred onto a new 24-well plate in 2 mL DC medium and incubated with the following stimuli in vitro: 100 ng/mL LPS (Escherichia coli O26:B6; Sigma), 10 μg/mL PGN (Staphylococcus aureus; Sigma), 1 nM phosphorothioate-stabilized CpG ODN (oligodeoxynucleotide; 5′-TCCATGACGTTCCTGATGCT-3′; Integrated DNA Technologies, Coralville, IA), 100 μg/mL poly (I:C) (Amersham Biosciences, Freiburg, Germany), and live or UV-VV (MOI of 1). The cells were then incubated for an additional 18 hours at 37°C. Supernatants were analyzed for cytokine production by enzyme-linked immunosorbent assay (ELISA).
Cytokine measurements by ELISA
Production of IL-1, IL-6, IL-12, IFN-α, and IFN-β by the DCs in response to various stimuli was detected in culture supernatants or sera by ELISA kits according to the manufacturers' standard protocols. IL-1β, IL-6, and IL-12p70 ELISA kits were purchased from Endogen Pierce (Woburn, MA), and IFN-α and IFN-β were obtained from PBL Biomedical Laboratories (Picataway, NJ).
Proliferation assay
T cells were isolated from splenocytes using CD5-microbeads (Miltenyi Biotec, Auburn, CA). CD5+ T cells (2 × 105) were cocultured with irradiated (3000 rad) naive splenocytes (2 × 105) in graded doses (MOI of 0.02, 0.1, or 0.5) of VV for 72 hours in 200 μL RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 50 μM β-mercaptoethanol, 100 IU/mL penicillin, and 100 IU/mL streptomycin in 96-well U-bottom tissue-culture plates, in triplicate. Cultures were pulsed with 1 μCi (0.037 MBq) per well of 3H-thymidine. At 16 to 20 hours after 3H-thymidine pulsing, the plates were harvested using a 96-well cell harvester and the 3H-thymidine incorporation was counted using a 1205 Betaplate liquid scintillation counter (Wallac, Ramsey, MN).
Antibodies and flow cytometry
FITC-conjugated anti-CD11c, FITC-conjugated anti–IFN-γ, PE-conjugated anti-CD86, and PE-Cy5–conjugated anti-CD8 antibodies were purchased from BD Pharmingen (San Diego, CA). FACScan (Becton Dickinson, Franklin Lakes, NJ) was used for flow cytometry event collection, which was analyzed using CELLQuest software (Becton Dickinson).
Intracellular cytokine staining
To assess production of IFN-γ intracellularly, splenocytes were incubated with 1 μg/mL anti-CD3 and anti-CD28 (BD Pharmingen) antibodies and 5 μg/mL Brefeldin A containing Golgi-Plug (BD Pharmingen) for 4 hours at 37°C. Intracellular cytokine staining was performed as previously described.11 Briefly, the cells were first stained with anti-CD8 followed by fixation and permeabilization with Cytofix/Cytoperm (BD Pharmingen). The cells were subsequently stained intracellularly with anti–IFN-γ. Samples were acquired on a BD Biosciences FACSCalibur flow cytometer and analysis was performed using CellQuest software (BD Biosciences).
Ovary VV titer assay
Viral load in the ovaries was measured by plaque-forming assay as described.21 Briefly, mice were killed 3 days after infection, and ovaries were harvested and stored at −80°C. Ovaries from individual mice were homogenized, freeze-thawed 3 times, and sonicated. Serial dilutions were performed and the virus titers were then determined by plaque assay on confluent TK-143B cells.
Statistical analysis
Results are expressed as the mean plus or minus the standard deviation (SD). Differences between groups were examined for statistical significance using student t test.
Results
Vaccinia virus stimulates DCs to secrete proinflammatory cytokines and IFN-β
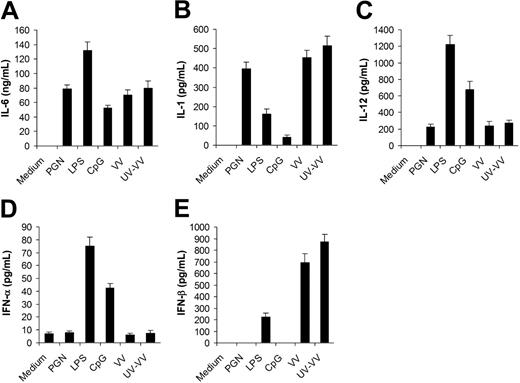
To study innate immune response to VV, we first compared the profile of cytokines secreted by in vitro–cultured DCs upon VV infection with that elicited by different TLR ligands. DCs were generated from bone marrow cells in the presence of GM-CSF and IL-4. After 5 days of culture, immature DCs were stimulated with PGN (TLR2 ligand), LPS (TLR4 ligand), CpG (TLR9 ligand), live VV or UV-VV at an MOI of 1. Supernatants were analyzed 18 hours later for secretion of proinflammatory cytokines, including IL-6, IL-1, and IL-12, as well as type I IFNs including IFN-α and IFN-β. DCs infected with VV produced high levels of IL-6 (Figure 1A) and IL-1 (Figure 1B), a detectable level of IL-12 (Figure 1C), but no IFN-α (Figure 1D). This pattern of cytokine production was very similar to that of PGN, but not LPS or CpG. However, very different from PGN was that VV also induced high levels of IFN-β (Figure 1E). These results indicated that VV could stimulate DCs to produce both proinflammatory cytokines and IFN-β, but not IFN-α. The induction of cytokine production by VV appeared to be independent of newly synthesized viral gene products after infection as similar levels of cytokines were induced by UV-VV (Figure 1).
Secretion of proinflammatory cytokines and IFN-β by DCs stimulated with vaccinia virus (VV). DCs were generated from bone marrow cells of C57BL/6 mice in the presence of GM-CSF and IL-4 for 5 days. On day 5, immature DCs were stimulated with PGN, LPS, CpG ODN (CpG), live VV, or UV-inactivated VV (UV-VV) for 18 hours. DCs incubated with medium only serve as the control (medium). Culture supernatants were analyzed by ELISA for secretion of IL-6 (A), IL-1 (B), IL-12 (C), IFN-α (D), and IFN-β (E). Results are expressed as mean ± SD.
Secretion of proinflammatory cytokines and IFN-β by DCs stimulated with vaccinia virus (VV). DCs were generated from bone marrow cells of C57BL/6 mice in the presence of GM-CSF and IL-4 for 5 days. On day 5, immature DCs were stimulated with PGN, LPS, CpG ODN (CpG), live VV, or UV-inactivated VV (UV-VV) for 18 hours. DCs incubated with medium only serve as the control (medium). Culture supernatants were analyzed by ELISA for secretion of IL-6 (A), IL-1 (B), IL-12 (C), IFN-α (D), and IFN-β (E). Results are expressed as mean ± SD.
Production of proinflammatory cytokines is dependent on MyD88, whereas secretion of IFN-β is TLR independent
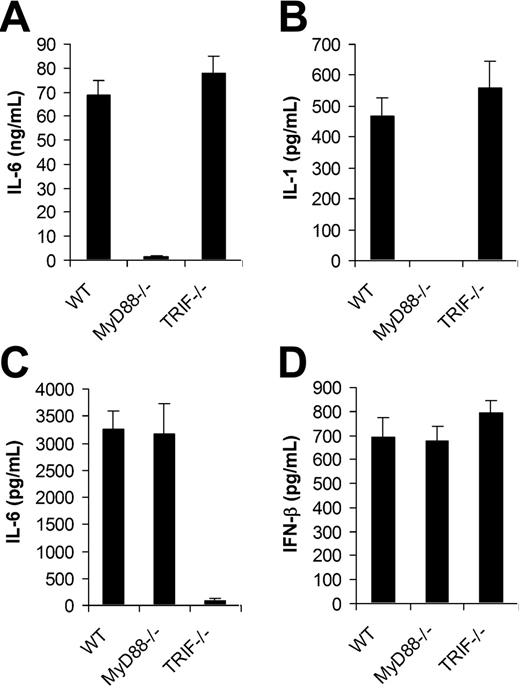
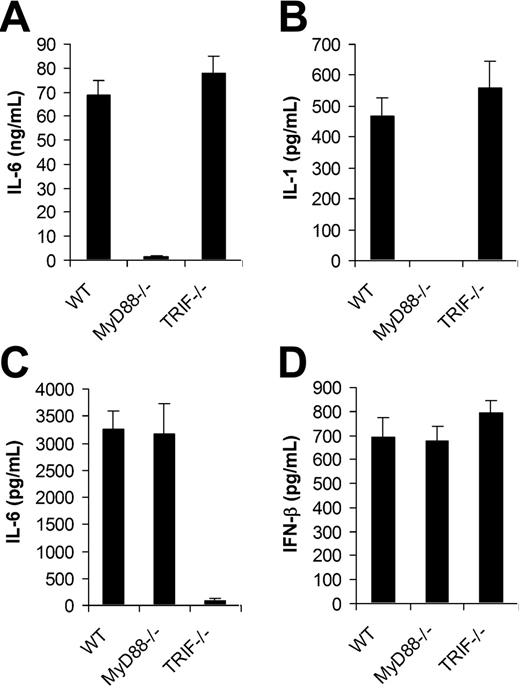
We next examined if TLRs were involved in the recognition of VV by DCs. Since all TLR signaling is mediated by MyD88 and/or TRIF,12 DCs deficient for MyD88 (MyD88−/−) or TRIF (TRIF−/−) were tested for their ability to produce proinflammatory cytokines and IFN-β upon VV infection. MyD88−/− and TRIF−/− DCs were first produced from bone marrow cells of MyD88−/− and TRIF−/− mice, respectively. Similar percentages of CD11c+ DCs were generated from MyD88−/− or TRIF−/− mice compared with the wild-type (WT) control after 5 days of culture (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article), suggesting MyD88 or TRIF deficiency did not affect the efficiency of DC differentiation in vitro. The same numbers of CD11c+ DCs from each group were stimulated with VV for 18 hours and the supernatants were assayed for the production of IL-6, IL-1, and IFN-β. MyD88−/− DCs stimulated with VV showed greatly diminished levels of IL-6 (Figure 2A) and IL-1 (Figure 2B), whereas the production of IL-6 and IL-1 was not affected by the TRIF−/− DCs compared with WT DCs. By contrast, when stimulated with TLR3 ligand poly (I:C), MyD88−/− DCs produced similar levels of IL-6 compared with the WT control (Figure 2C), whereas TRIF−/− DCs produced little IL-6. This was consistent with the previous observation that cytokine production mediated by TLR3 is TRIF dependent but MyD88 independent, and suggested the lack of IL-6 production by VV-stimulated MyD88−/− DCs was not due to their inherent defect in cytokine production. Surprisingly, in contrast to IL-6 production, both MyD88−/− and TRIF−/− DCs secreted similar levels of IFN-β upon VV infection compared with WT DCs (Figure 2D). These data indicated that VV-induced production of proinflammatory cytokines was TLR mediated and dependent on MyD88, whereas the induction of IFN-β was independent of TLRs.
MyD88 is required for the induction of proinflammatory cytokines by VV, whereas secretion of IFN-β is independent of MyD88 or TRIF. DCs were generated from bone marrow cells of wild-type C57BL/6 (WT), MyD88−/− or TRIF−/− mice in the presence of GM-CSF and IL-4 for 5 days. On day 5, immature DCs were stimulated with VV at an MOI of 1 for 18 hours. Supernatants were then analyzed by ELISA for secretion of IL-6 (A), IL-1 (B), and IFN-β (D). DCs were also stimulated with poly (I:C) for 18 hours and assayed for secretion of IL-6 (C). Results are expressed as mean ± SD.
MyD88 is required for the induction of proinflammatory cytokines by VV, whereas secretion of IFN-β is independent of MyD88 or TRIF. DCs were generated from bone marrow cells of wild-type C57BL/6 (WT), MyD88−/− or TRIF−/− mice in the presence of GM-CSF and IL-4 for 5 days. On day 5, immature DCs were stimulated with VV at an MOI of 1 for 18 hours. Supernatants were then analyzed by ELISA for secretion of IL-6 (A), IL-1 (B), and IFN-β (D). DCs were also stimulated with poly (I:C) for 18 hours and assayed for secretion of IL-6 (C). Results are expressed as mean ± SD.
TLR2 is required for VV-induced secretion of proinflammatory cytokines
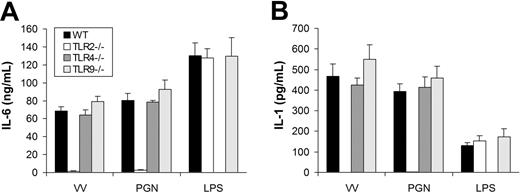
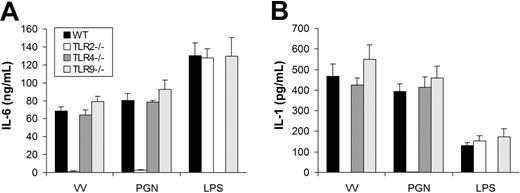
We next determined which TLR mediated the MyD88-dependent production of proinflammatory cytokines. The similarity in the pattern of cytokine secretion between TLR2 ligand PGN and VV suggested TLR2 as a likely candidate to mediate VV-induced production of proinflammatory cytokines. To test this hypothesis, we examined whether DCs defective for TLR2 (TLR2−/−) secreted proinflammatory cytokines in response to VV infection. Like MyD88 or TRIF deficiency, TLR2 deficiency did not appear to alter DC differentiation in vitro as similar percentages of CD11c+ DCs were generated from TLR2−/− mice compared with the WT control (Figure S1). Similar to TLR2−/− DCs treated with PGN, TLR2−/− DCs stimulated with VV failed to secrete IL-6 (Figure 3A) or IL-1 (Figure 3B), whereas the production of IL-6 or IL-1 was not affected in TLR4−/− or TLR9−/− DCs in comparison to WT DCs upon VV infection. The lack of proinflammatory cytokine production by VV-stimulated TLR2−/− DCs was not due to their inherent inability to secrete cytokines as TLR2−/− DCs stimulated with TLR4 ligand LPS produced similar levels of IL-6 (Figure 3A) or IL-1 (Figure 3B) compared with the WT control. These data showed that induction of proinflammatory cytokines by VV was mediated through TLR2.
VV-induced production of proinflammatory cytokines is mediated by TLR2. DCs were generated from bone marrow cells of wild-type C57BL/6 (WT), TLR2−/−, TLR4−/−, or TLR9−/− mice in the presence of GM-CSF and IL-4 for 5 days. On day 5, immature DCs were stimulated with VV, PGN, or LPS for 18 hours. Supernatants were then analyzed by ELISA for secretion of IL-6 (A) and IL-1 (B). Error bars indicate SD.
VV-induced production of proinflammatory cytokines is mediated by TLR2. DCs were generated from bone marrow cells of wild-type C57BL/6 (WT), TLR2−/−, TLR4−/−, or TLR9−/− mice in the presence of GM-CSF and IL-4 for 5 days. On day 5, immature DCs were stimulated with VV, PGN, or LPS for 18 hours. Supernatants were then analyzed by ELISA for secretion of IL-6 (A) and IL-1 (B). Error bars indicate SD.
TLR2/MyD88 pathway is required for innate and adaptive immunity to vaccinia virus in vivo
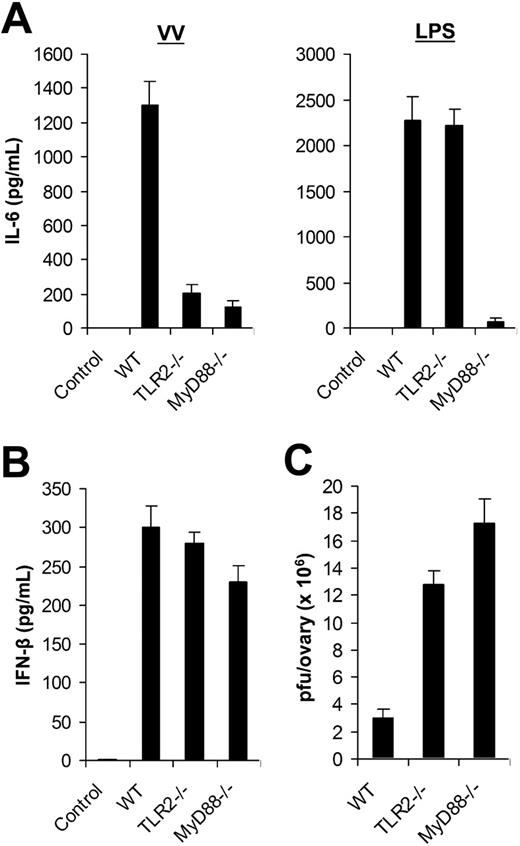
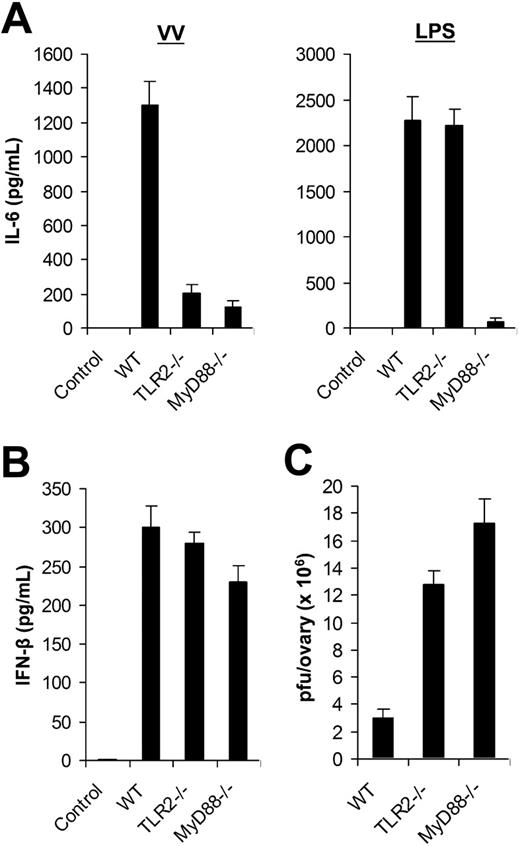
Using DCs generated from bone marrow cells in vitro, we identified that VV-induced production of proinflammatory cytokines was mediated through TLR2 in a MyD88-dependent fashion. Here we examined the in vivo relevance of VV recognition by TLR2. WT, TLR2−/−, or MyD88−/− mice were injected with 1 × 107 pfu of VV intravenously. Serum was harvested 6 hours later and assayed for IL-6 secretion by ELISA. Serum from WT mice injected with VV was found to contain significant levels of IL-6, whereas no IL-6 secretion was detected in the uninfected WT mice (Figure 4A). However, IL-6 levels were significantly reduced in VV-infected TLR2−/− and MyD88−/− mice (P < .001; Figure 4A). Similar to the in vitro studies (Figure 3), there was no inherent defect in producing IL-6 by TLR2−/− mice in vivo as TLR2−/− mice treated with LPS secreted similar levels of IL-6 compared with the WT mice (Figure 4A). Thus, the secretion of IL-6 upon VV infection in vivo also required an intact TLR2 signaling pathway. By contrast, the production of IFN-β was independent of TLR2 or MyD88 as comparable levels of IFN-β were detected in TLR2−/− or MyD88−/− mice compared with the WT mice upon VV infection in vivo (Figure 4B).
The TLR2/MyD88-dependent pathway is required for innate immune control of VV infection in vivo. (A) Production of IL-6. Wild-type C57BL/6 (WT), TLR2−/−, or MyD88−/− mice were administered intravenously with 1 × 107 pfu VV or 30 μg LPS, and serum samples were harvested 6 hours later for secretion of IL-6 by ELISA. Serum from uninfected C57BL/6 mice was used as control (control). (B) Secretion of IFN-β. WT, TLR2−/−, or MyD88−/− mice were injected intravenously with VV and serum samples were assayed for secretion of IFN-β. Uninfected C57BL/6 mice were used as control (control). (C) Viral titer. 1 × 107 pfu VV was injected intraperitoneally into female WT, TLR2−/−, or MyD88−/− mice and ovaries were harvested for measurement of viral load by plaque-forming assay 3 days later. Data represent viral titer as plaque-forming unit (pfu) per ovary. Error bars indicate SD.
The TLR2/MyD88-dependent pathway is required for innate immune control of VV infection in vivo. (A) Production of IL-6. Wild-type C57BL/6 (WT), TLR2−/−, or MyD88−/− mice were administered intravenously with 1 × 107 pfu VV or 30 μg LPS, and serum samples were harvested 6 hours later for secretion of IL-6 by ELISA. Serum from uninfected C57BL/6 mice was used as control (control). (B) Secretion of IFN-β. WT, TLR2−/−, or MyD88−/− mice were injected intravenously with VV and serum samples were assayed for secretion of IFN-β. Uninfected C57BL/6 mice were used as control (control). (C) Viral titer. 1 × 107 pfu VV was injected intraperitoneally into female WT, TLR2−/−, or MyD88−/− mice and ovaries were harvested for measurement of viral load by plaque-forming assay 3 days later. Data represent viral titer as plaque-forming unit (pfu) per ovary. Error bars indicate SD.
We then asked if the TLR2/MyD88 pathway was required for innate immune control of VV infection in vivo. VV (1 × 107 pfu) was injected intraperitoneally into female WT, TLR2−/−, or MyD88−/− mice. Three days later, the viral load in the ovaries was determined by the plaque assay. Significantly (P < .001) higher VV titer was detected in TLR-2−/− or MyD88−/− mice than in the WT control (Figure 4C). As adaptive immunity against VV had yet to be elicited at 3 days after infection (data not shown), these results suggested that the TLR2/MyD88 pathway was critical in innate immune control of VV infection in vivo.
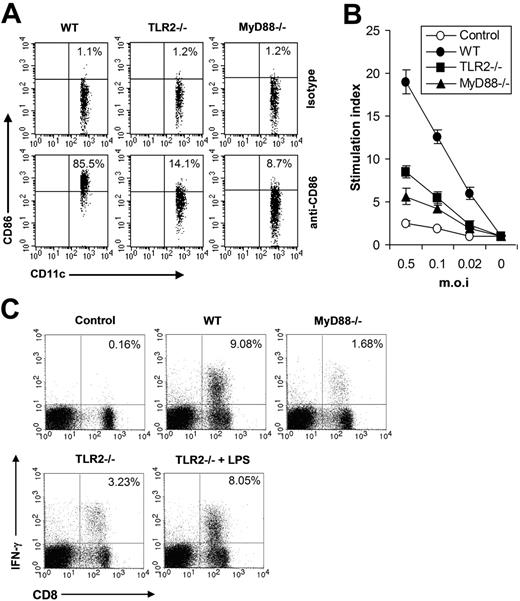
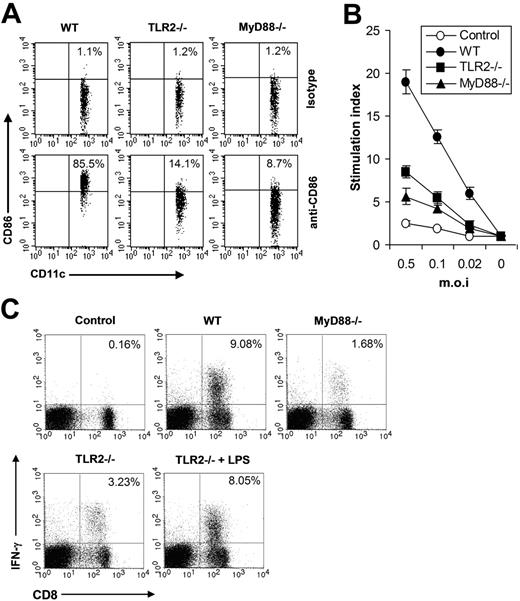
Next we investigated the functional significance of the TLR2-mediated innate immune pathway in vivo in shaping adaptive immune response to VV. As DC maturation is a key initial event in triggering adaptive immune response to pathogenic infections, we first examined the maturation of splenic CD11c+ DCs in WT, TLR2−/−, or MyD88−/− mice 24 hours after VV infection. The result showed that the percentage of CD11c+ DCs expressing CD86 was significantly (P < .001) reduced in TLR2−/− or MyD88−/− mice (Figure 5A), indicating that the TLR2/MyD88 signaling pathway was required for promoting DC maturation in vivo upon VV infection. Seven days after VV infection, T cells isolated from splenocytes were analyzed for virus-induced T-cell activation using 3H-thymidine incorporation assay upon in vitro restimulation with different doses of VV. In WT mice, VV infection resulted in robust T-cell activation, whereas in TLR2−/− or MyD88−/− mice T-cell activation was significantly diminished (P < .001; Figure 5B). Splenocytes were also analyzed for virus-specific CD8 effectors using IFN-γ intracellular staining. In VV-infected WT mice, about 9% of total lymphocytes are IFN-γ–secreting CD8 T cells, compared with only 0.16% in uninfected mice (Figure 5C). However, in TLR2−/− or MyD88−/− mice, the percentage of effector CD8 T cells was significantly reduced (P < .001, Figure 5C). Co-administration of LPS to TLR2−/− mice significantly increased the percentage of effector CD8 T cells (P < .01; Figure 5C), suggesting there was no inherent defect in generating VV-specific T-cell response in TLR2−/− mice. Taken together, these data indicated that the TLR2/MyD88 innate immune pathway was required for efficient adaptive immune response to VV infection in vivo.
The TLR2/MyD88 signaling pathway promotes adaptive immune response to VV in vivo (A) Maturation of splenic CD11c+ DCs. Wild-type C57BL/6 (WT), TLR2−/−, or MyD88−/− were administered intravenously with 1 × 107 pfu VV and splenocytes were harvested 24 hours later, stained with anti-CD11c and anti-CD86 or an isotype control antibody (isotype), and analyzed for CD86 expression by FACS based on isotype control antibody staining on respective CD11c+ cells. The percentages of CD11c+ DCs expressing CD86 are indicated. (B) VV-specific T-cell activation. Seven days after infection, CD5+ T cells purified from splenocytes were restimulated with VV at an MOI of 0.5, 0.1, or 0.02 in the presence of corresponding naive irradiated splenocytes. Proliferation of virus-specific T cells was analyzed by 3H-thymidine incorporation. Data reflect the mean plus or minus SD of the stimulation index, calculated by dividing 3H counts in cpm in the presence of viral stimulation by those in the absence of stimulation, as a function of different virus doses. T cells harvested from uninfected C57BL/6 mice were used as control (control). (C) Activation of VV-specific effector CD8 T cells. Seven days after VV infection, splenocytes were stimulated with anti-CD3 and anti-CD28 for 4 hours and assayed for intracellular IFN-γ secretion by CD8 T cells. Splenocytes from uninfected C57BL/6 mice were used as control (control). In some TLR2−/− mice, 30 μg LPS was co-injected at time of VV infection (TLR2−/− plus LPS). Events were gated on total lymphocytes. The percentages of IFN-γ–producing CD8 T cells among total lymphocytes are indicated.
The TLR2/MyD88 signaling pathway promotes adaptive immune response to VV in vivo (A) Maturation of splenic CD11c+ DCs. Wild-type C57BL/6 (WT), TLR2−/−, or MyD88−/− were administered intravenously with 1 × 107 pfu VV and splenocytes were harvested 24 hours later, stained with anti-CD11c and anti-CD86 or an isotype control antibody (isotype), and analyzed for CD86 expression by FACS based on isotype control antibody staining on respective CD11c+ cells. The percentages of CD11c+ DCs expressing CD86 are indicated. (B) VV-specific T-cell activation. Seven days after infection, CD5+ T cells purified from splenocytes were restimulated with VV at an MOI of 0.5, 0.1, or 0.02 in the presence of corresponding naive irradiated splenocytes. Proliferation of virus-specific T cells was analyzed by 3H-thymidine incorporation. Data reflect the mean plus or minus SD of the stimulation index, calculated by dividing 3H counts in cpm in the presence of viral stimulation by those in the absence of stimulation, as a function of different virus doses. T cells harvested from uninfected C57BL/6 mice were used as control (control). (C) Activation of VV-specific effector CD8 T cells. Seven days after VV infection, splenocytes were stimulated with anti-CD3 and anti-CD28 for 4 hours and assayed for intracellular IFN-γ secretion by CD8 T cells. Splenocytes from uninfected C57BL/6 mice were used as control (control). In some TLR2−/− mice, 30 μg LPS was co-injected at time of VV infection (TLR2−/− plus LPS). Events were gated on total lymphocytes. The percentages of IFN-γ–producing CD8 T cells among total lymphocytes are indicated.
TLR-independent production of IFN-β is also critical for innate and adaptive immunity against VV in vivo
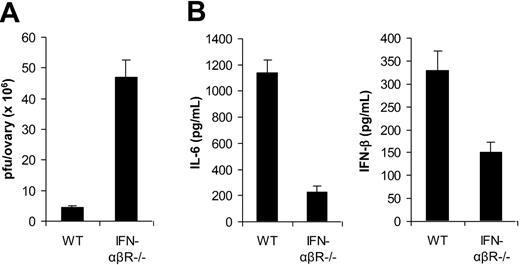
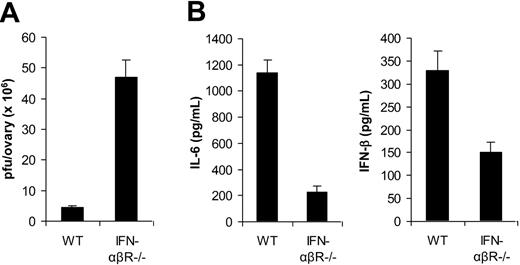
In addition to TLR2/MyD88-dependent induction of proinflammatory cytokines, VV also triggered secretion of IFN-β through a TLR-independent pathway. Here we investigated the biologic significance of VV-induced, TLR-independent production of IFN-β in vivo. Given that type I IFNs such as IFN-β have been shown to play an important role in innate antiviral defense,22 we first examined the role of IFN-β in innate immune control of VV infection in vivo. 1 × 107 pfu of VV was injected intraperitoneally into female WT mice or mice deficient for the IFN-α and IFN-β receptor (IFN-αβR−/−). Three days later, the viral load in the ovaries was determined by the plaque assay. Significantly (P < .001) higher VV titer was detected in IFN-αβR−/− than in WT mice (Figure 6A). These results suggested that VV-induced IFN-β was also critical in innate immune control of VV infection in vivo.
Innate immunity to VV is dependent on TLR-independent induction of IFN-β. (A) Innate antiviral defense. VV (1 × 107 pfu) was injected intraperitoneally into female, wild-type 129/Sv mice (WT) or IFN-αβR−/− mice on 129/Sv background (IFN-αβR−/−). Ovaries were harvested 3 days after administration of virus and measured for viral load by plaque-forming assay. Data represent viral titer as plaque-forming unit (pfu) per ovary. (B) Cytokine production. WT or IFN-αβR−/− mice were administered intravenously with 1 × 107 pfu VV, and serum samples were harvested 6 hours later for the measurement of IL-6 and IFN-β by ELISA. Error bars indicate SD.
Innate immunity to VV is dependent on TLR-independent induction of IFN-β. (A) Innate antiviral defense. VV (1 × 107 pfu) was injected intraperitoneally into female, wild-type 129/Sv mice (WT) or IFN-αβR−/− mice on 129/Sv background (IFN-αβR−/−). Ovaries were harvested 3 days after administration of virus and measured for viral load by plaque-forming assay. Data represent viral titer as plaque-forming unit (pfu) per ovary. (B) Cytokine production. WT or IFN-αβR−/− mice were administered intravenously with 1 × 107 pfu VV, and serum samples were harvested 6 hours later for the measurement of IL-6 and IFN-β by ELISA. Error bars indicate SD.
We then studied the role of IFN-β in TLR2-dependent production of proinflammatory cytokines. WT or IFN-αβR−/− mice were infected with VV and 6 hours later serum was measured for IL-6 and IFN-β secretion by ELISA. Our results showed that the production of IL-6 was significantly (P < .001) diminished in IFN-αβR−/− mice compared with the WT control (Figure 6B), suggesting IFN-β is necessary for TLR2-dependent induction of proinflammatory cytokines by VV in vivo. Furthermore, we found that the secretion of IFN-β was also reduced in IFN-αβR−/− mice (Figure 6B), indicating that IFN-β signaling was also required for efficient production of type I IFNs through an autocrine-paracrine loop, as suggested.23
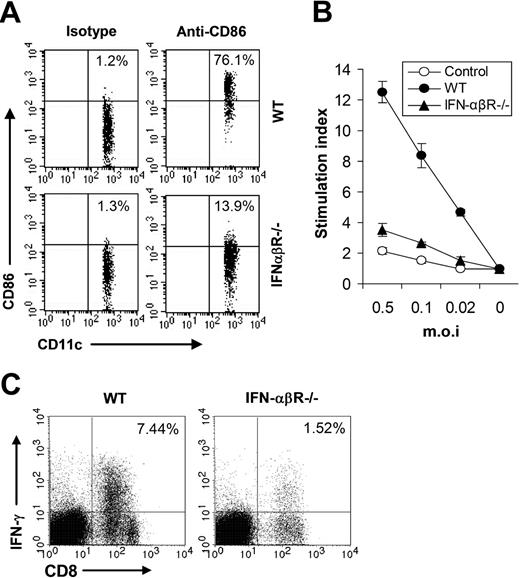
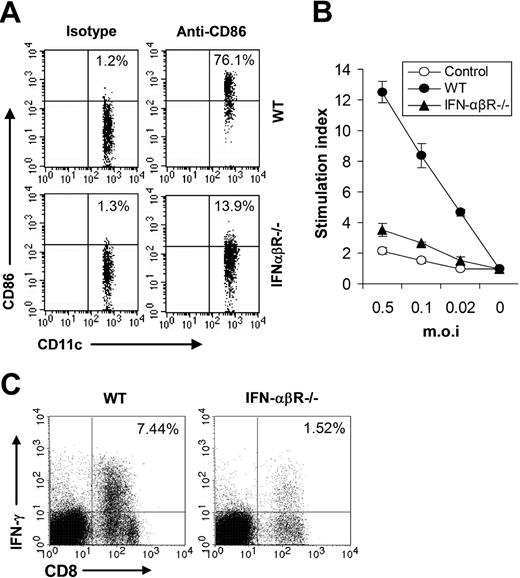
We next sought to study whether IFN-β also contributed to adaptive immune response to VV infection in vivo. We first examined the maturation of splenic CD11c+ DCs in WT or IFN-αβR−/− mice 24 hours after VV infection and the result showed that the percentage of CD11c+ DCs expressing CD86 was greatly reduced in IFN-αβR−/− mice (Figure 7A), suggesting IFN-β signaling was required for promoting DC maturation in vivo upon VV infection. Splenocytes from WT or IFN-αβR−/− mice were analyzed for virus-specific T-cell activation 7 days after VV infection, using 3H-thymidine incorporation assay upon in vitro restimulation with different doses of VV. As shown in Figure 7B, virus-specific T-cell activation was severely compromised in IFN-αβR−/− mice compared with the WT mice. In addition, in IFN-αβR−/− mice the percentage of effector CD8 T cells was also greatly reduced (Figure 7C). Taken together, these data indicated that VV-induced, TLR-independent production of IFN-β also played a pivotal role in innate and adaptive immune responses to VV infection in vivo.
IFN-β is required for efficient adaptive immunity against VV in vivo. (A) Maturation of splenic CD11c+ DCs. Wild-type 129/Sv (WT) or IFN-αβR−/− mice were administered 1 × 107 pfu VV intravenously, and splenocytes were harvested 24 hours later, stained with anti-CD11c and anti-CD86 or an isotype control antibody (isotype), and analyzed for CD86 expression by FACS based on isotype control antibody staining on respective CD11c+ cells. The percentages of CD11c+ DCs expressing CD86 are indicated. (B) VV-specific T-cell activation. Seven days after VV infection, CD5+ T cells purified from splenocytes were restimulated with VV at an MOI of 0.5, 0.1, or 0.02 in the presence of corresponding naive irradiated splenocytes. Proliferation of virus-specific T cells was analyzed by 3H-thymidine incorporation. Data reflect the mean plus or minus SD of the stimulation index, calculated by dividing 3H counts in cpm in the presence of viral stimulation by those in the absence of stimulation, as a function of different virus doses. T cells from uninfected 129/Sv mice were used as control (control). (C) Activation of VV-specific effector CD8 T cells. Seven days after infection, splenocytes were stimulated with anti-CD3 and anti-CD28 for 4 hours and assayed for intracellular IFN-γ secretion by CD8 T cells. Events were gated on total lymphocytes. The percentages of IFN-γ–producing CD8 T cells among total lymphocytes are indicated.
IFN-β is required for efficient adaptive immunity against VV in vivo. (A) Maturation of splenic CD11c+ DCs. Wild-type 129/Sv (WT) or IFN-αβR−/− mice were administered 1 × 107 pfu VV intravenously, and splenocytes were harvested 24 hours later, stained with anti-CD11c and anti-CD86 or an isotype control antibody (isotype), and analyzed for CD86 expression by FACS based on isotype control antibody staining on respective CD11c+ cells. The percentages of CD11c+ DCs expressing CD86 are indicated. (B) VV-specific T-cell activation. Seven days after VV infection, CD5+ T cells purified from splenocytes were restimulated with VV at an MOI of 0.5, 0.1, or 0.02 in the presence of corresponding naive irradiated splenocytes. Proliferation of virus-specific T cells was analyzed by 3H-thymidine incorporation. Data reflect the mean plus or minus SD of the stimulation index, calculated by dividing 3H counts in cpm in the presence of viral stimulation by those in the absence of stimulation, as a function of different virus doses. T cells from uninfected 129/Sv mice were used as control (control). (C) Activation of VV-specific effector CD8 T cells. Seven days after infection, splenocytes were stimulated with anti-CD3 and anti-CD28 for 4 hours and assayed for intracellular IFN-γ secretion by CD8 T cells. Events were gated on total lymphocytes. The percentages of IFN-γ–producing CD8 T cells among total lymphocytes are indicated.
Discussion
In this paper, we demonstrated that VV activated innate immunity through a TLR2/MyD88-dependent pathway, resulting in the production of proinflammatory cytokines, and a TLR-independent pathway, leading to the induction of IFN-β in vitro and in vivo. More importantly, both innate immune pathways were critical for innate and adaptive immunity against VV infection in vivo.
In addition to the functional significance of TLRs in innate recognition of bacteria and fungi, recent studies have demonstrated a crucial role of the TLRs in mediating antiviral defense, such as TLR4 in respiratory syncytial virus (RSV) infection,18 TLR3 in double-stranded RNA viral infection,24 TLR7 and TLR8 in single-stranded RNA viruses,25,26 and TLR9 in herpes simplex virus (HSV) infection.27,28 Our data provide the first evidence that innate immune recognition of VV is mediated by TLR2 in a MyD88-dependent manner. The biologic significance of this innate immune pathway lies in its critical role in both innate and adaptive immunity to VV in vivo. The mechanism(s) underlying TLR2/MyD88-dependent innate immune control of VV infection in vivo remains to be further defined. However, it appears not to be mediated by type I IFNs since no significant reduction of IFN-β was observed in TLR-2−/− or MyD88−/− mice (Figure 4B). Similarly, besides promoting DC maturation (Figure 5A), it is not entirely clear how the TLR2/MyD88 pathway contributes to the activation of adaptive immunity to VV. Recent observations by others and by our group suggest that this may be mediated through TLR2-induced proinflammatory cytokines such as IL-6 and IL-1, which render naive T cells refractory to TReg-mediated suppression.11,29,30 Another question that remains to be answered is which component(s) of VV activates the TLR2 signaling pathway. Our observation that UV-VV elicited similar response to live VV suggests that input VV envelope or core protein(s) rather than newly synthesized viral product after infection may trigger the TLR2 pathway. Thus, it will be important to identify the envelope or core protein(s) of VV that activates the TLR2 pathway. Identification of such a protein(s) will help in the design of effective vaccines.
We further demonstrated that VV also activated a TLR-independent pathway leading to production of IFN-β. In addition to TLR-dependent innate immune recognition, accumulating evidence has shown the existence of TLR-independent recognition of pathogens.13 It has been shown that viral dsRNA can activate innate immunity mediated by an intracellular sensor, RIG-1, leading to the production of type I IFNs through an IPS-1/TBK1-dependent but TLR3-independent pathway.31 In addition, dsDNA can also trigger the production of type I IFNs in a TLR-independent manner.32,33 Furthermore, NOD (nucleotide binding oligomerization domain)–containing proteins have been implicated in the recognition of bacteria independent of TLRs, leading to activation of caspase-1, which promotes the conversion of procytokines such as pro-IL-1β to mature cytokines.34 This process is catalyzed by a complex of proteins designated as inflammasome.35 It is not clear whether the TLR-independent recognition of VV is mediated by viral DNA or other mechanisms. Thus, future studies should focus on delineation of the mechanisms underlying TLR-independent recognition of VV.
In vivo, plasmacytoid DCs (pDCs) have been shown to produce large amounts of IFN-α mediated by TLR7, TLR8, or TLR9 in response to various viral infections.36 However, our observation that innate immune recognition of VV is mediated by TLR2 and that production of IFN-β is TLR independent suggest that pDCs are unlikely the producers of IFN-β upon VV infection. Indeed, recent studies have shown that conventional DCs and macrophages can become potent producers of type I IFNs upon viral infection.37,38 Our data that bone marrow–derived DCs secrete high levels of IFN-β in response to VV are in line with these observations.
Although type I IFNs such as IFN-β have been shown to play an important role in innate antiviral defense,22 their precise role in innate immune control of VV infection has not been determined. Our study clearly shows that IFN-β is required for not only the innate immune control of VV infection, but also the TLR2/MyD88-dependent production of proinflammatory cytokines. In addition, IFN-β also plays a pivotal role in adaptive immune responses to VV. Although TLR2/MyD88-dependent activation of adaptive immunity to VV in vivo is independent of IFN-β, type I IFN signaling is required for the efficient production of IL-6 (Figure 6B). Thus, IFN-β could contribute to the activation of adaptive immunity to VV indirectly through enhancing the TLR2/MyD88-dependent production of proinflammatory cytokines. However, previous studies have suggested that type I IFNs could also contribute directly to activation of adaptive immune response against VV. First, it has been shown that type I IFNs induce DC maturation by up-regulating the expression of costimulatory molecules, which in turn leads to efficient homing to secondary lymphoid organs and priming of CD8+ and CD4+ T-cell responses.39,40 Second, DCs matured by type I IFNs promote cross-priming of virus-specific CD8+ T cells.41 Third, type I IFNs can promote effector function of virus-specific CD8+ T cells,42,43 and enhance the survival of activated T cells.44-46 Finally, a recent report indicates that type I IFN signaling directly through CD8+ T cells is required for clonal expansion in response to viral infection in vivo.47
In conclusion, we have shown that VV elicits innate immune response through the TLR2/MyD88-dependent pathway, which plays a critical role in activating innate and adaptive immunity to VV. We have further identified that VV also triggers a TLR-independent pathway leading to the production of IFN-β, which is essential for effective innate and adaptive immune responses to VV in vivo. These results indicate that one pathogen can target both TLR and non-TLR innate immune pathways to maximize activation of host defense and suggest potential new strategies for the design of effective vaccines.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: J.Z., J.M., and X.H. performed research, and collected and analyzed data; and Y.Y. designed research, analyzed data, and wrote the paper.
Acknowledgments
We thank Dr Shizuo Akira for providing TLR2−/−, TLR9−/−, MyD88−/−, and TRIF−/− mice.
This work was supported by grants CA111807 and CA047741 from the National Institutes of Health, and the Investigator Award from the Alliance for Cancer Gene Therapy (Y.Y.).