Abstract
The threat from cancer cells is inherently linked to cell-cycle progression, and viral genomes commonly replicate, for example, within episomes or proviruses, during mitosis. We report here that human natural killer (NK) cells bound cells in mitosis and attacked pathogenic cells in mitosis more effectively than the same cells in other stages of the cell cycle. Thus, cells in mitosis warrant and undergo heightened surveillance, a novel strategy for immunologic assessment of danger. Recognition of cells in mitosis involved ligation of activating NK-cell receptors and binding to target-cell hyaluronan, a component of the pericellular matrix known to be increased during mitosis. Direct interaction between activating NK-cell receptors and hyaluronan is possible, but other mechanisms consistent with our data are also discussed.
Introduction
Viral-infected or tumor cells can become susceptible to lysis by natural killer (NK) cells via expression of stress-inducible proteins1 or by decreased expression of self class I MHC protein.2 Many viruses, including human cytomegalovirus known to be targeted by NK-cell surveillance, encode genes that help drive cell-cycle progression.3 In addition, NK cells react more efficiently to concanavalin A-stimulated, proliferating MHC class I–deficient target cells than to nonactivated cells in vitro and in vivo.4,5 However, whether cell-cycle progression could be used as an endogenous indicator for potential danger has not been considered. To test this, we compared the innate response of human NK cells to target cells in mitosis or other stages of the cell cycle.
Materials and methods
Cells and imaging
Human peripheral blood NK cells were isolated as described previously.6 For T cells, peripheral blood mononuclear cells (PBMCs) were stained with FITC-labeled anti-CD3 monoclonal antibody (mAb; (BD Biosciences, San Jose, CA), positively enriched using anti-FITC microbeads (Miltenyi Biotec, Auburn, CA), and cultured for 1 week in IL-2–containing medium. Peripheral blood NK cells and target cells were coincubated at 37°C/5% CO2, fixed, stained, and imaged.6 Cells were stained using anti–α-tubulin mAb (clone DM1A; Sigma) used at 0.5 μL per 3 × 103 cells, followed by Alexa Fluor 488–labeled goat anti–mouse IgG (Molecular Probes, Paisley, United Kingdom), and were then washed in PBS. The fraction of mitotic and nonmitotic target cells bound by one or more NK cells was then assessed by confocal microscopy (TCS SP2; Leica, Heidelberg, Germany) using a 63 ×/1.32 numerical aperture oil objective. Images were acquired and analyzed using Leica confocal software version 2.61. Data were analyzed by multinomial logistic regression analysis with fixed model design (SPSS, Chicago, IL).
Enzyme treatment
Target cells were treated with 30 U/mL Streptomyces hyalurolyticus hyaluronidase, 0.5 U/mL chondroitinase ABC, or 1.5 U/mL heparinase I (Sigma, St Louis, MO) for 40 minutes. NK cells were preincubated with 1 μg/mL heparin (Leo Pharmaceuticals, High Wycombe, United Kingdom), heparan sulfate, chondroitin sulfate A or B, or hyaluronan (Sigma) for 30 minutes before coincubation.
Antibody-mediated blocking
NK cells were incubated for 30 minutes on ice with 10 μg/mL anti-CD44 blocking mAb or control isotype-matched anti-CD44 mAb (BRIC 235 or KZ1, respectively7 ), anti-NKp46 (195314; R&D Systems, Minneapolis, MN), mouse IgG2b control (BD Biosciences), goat polyclonal anti-NKp46 (C-20; R&D Systems), goat IgG control (Jackson ImmunoResearch, West Grove, PA), or anti-NKG2D (149810; R&D Systems). Anti-NKp44 mAb was a gift from Carsten Watzl (Heidelberg, Germany).
Enrichment for cells in mitosis
Target cells (721.221; 0.5 × 106/mL) were incubated overnight at 37°C, 5% CO2 with 0.1 μM colchicine (Sigma) or 100 ng/mL nocodazole (Sigma). For comparison, incubation in 10 μM colchicine for 1 hour was used. HT1080 cells were enriched by shake-off.8
Results and discussion
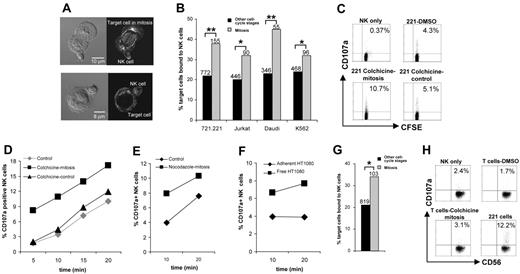
A variety of pathogenic target cells, namely, EBV-transformed MHC class I–deficient B cells 721.221, Jurkat T-cell leukemia cells, Burkitt lymphoma-derived Daudi cells, or myeloid leukemia K562 cells, were separately incubated with human NK cells derived from peripheral blood. Images of conjugates stained for α-tubulin were used to identify cells in mitosis by the characteristic mitotic spindle (Figure 1A). We found that NK cells conjugated to cells in mitosis significantly more frequently than to the same cells in other stages of the cell cycle (Figure 1B).
Evidence for increased surveillance of cells in mitosis by human NK cells. (A) Because no cell surface protein is known to be uniquely expressed during mitosis and flow cytometry using DNA dyes cannot specifically separate cells in mitosis, images of conjugates stained for α-tubulin were used to distinguish cells in mitosis (top row) from target cells in other stages of the cell cycle (bottom row). Micrographs show bright-field images (left column) and corresponding fluorescence images (right column) of NK cells (small cells) bound to target cells. (B) The percentage of various susceptible target cells bound by NK cells was assessed after 10 minutes of coincubation. Data from at least 3 independent experiments are shown, and the total numbers of cells indicated. (C-D) Target cells (721.221) were arrested in mitosis by incubation overnight with colchicine (▪) or treated with DMSO as a control (♦). Alternatively, 721.221 cells were treated with a higher concentration of colchicine for 1 hour to disrupt the cytoskeleton without arresting cells in mitosis (▴). Target cells were incubated with CFSE-labeled NK cells and the percentage of CD107a+ NK cells was determined by flow cytometry. (C) Representative flow cytometric dot plots after 10 minutes of coincubation. (D) Percentages of CD107a+ NK cells after different times of coincubation. (E) 721.221 target cells were arrested in mitosis by overnight incubation with nocodazole (▪) or the DMSO vehicle only, as a control (♦). Target cells were then incubated with NK cells, and the percentage of CD107a+ NK cells was determined by flow cytometry. (F) The fraction of HT1080 cells in mitosis was enriched using the shake-off method. The percentages of NK cells that were CD107a+ after incubation with free-floating HT1080 cells (containing ~40%-50% of cells in mitosis) or adherent HT1080 cells (in other stages of the cell cycle) were compared using flow cytometry. (G) The percent of autologous T cells bound by NK cells was assessed after 10 minutes of coincubation by imaging, as in panel B. (H) Autologous T cells were arrested in mitosis by overnight incubation with colchicine (~40% of cells in mitosis) or treated with DMSO as a control (3% of cells in mitosis). The percent of CD56+ NK cells that were CD107a+, that is, degranulated, was determined by flow cytometry after 1 hour of coincubation with T cells or positive control cells 721.221. (B,G) Data are from 3 independent experiments, and the total numbers of cells are indicated. (C-F,H) Representative data from 3 experiments are shown. *P <.05, **P <.005.
Evidence for increased surveillance of cells in mitosis by human NK cells. (A) Because no cell surface protein is known to be uniquely expressed during mitosis and flow cytometry using DNA dyes cannot specifically separate cells in mitosis, images of conjugates stained for α-tubulin were used to distinguish cells in mitosis (top row) from target cells in other stages of the cell cycle (bottom row). Micrographs show bright-field images (left column) and corresponding fluorescence images (right column) of NK cells (small cells) bound to target cells. (B) The percentage of various susceptible target cells bound by NK cells was assessed after 10 minutes of coincubation. Data from at least 3 independent experiments are shown, and the total numbers of cells indicated. (C-D) Target cells (721.221) were arrested in mitosis by incubation overnight with colchicine (▪) or treated with DMSO as a control (♦). Alternatively, 721.221 cells were treated with a higher concentration of colchicine for 1 hour to disrupt the cytoskeleton without arresting cells in mitosis (▴). Target cells were incubated with CFSE-labeled NK cells and the percentage of CD107a+ NK cells was determined by flow cytometry. (C) Representative flow cytometric dot plots after 10 minutes of coincubation. (D) Percentages of CD107a+ NK cells after different times of coincubation. (E) 721.221 target cells were arrested in mitosis by overnight incubation with nocodazole (▪) or the DMSO vehicle only, as a control (♦). Target cells were then incubated with NK cells, and the percentage of CD107a+ NK cells was determined by flow cytometry. (F) The fraction of HT1080 cells in mitosis was enriched using the shake-off method. The percentages of NK cells that were CD107a+ after incubation with free-floating HT1080 cells (containing ~40%-50% of cells in mitosis) or adherent HT1080 cells (in other stages of the cell cycle) were compared using flow cytometry. (G) The percent of autologous T cells bound by NK cells was assessed after 10 minutes of coincubation by imaging, as in panel B. (H) Autologous T cells were arrested in mitosis by overnight incubation with colchicine (~40% of cells in mitosis) or treated with DMSO as a control (3% of cells in mitosis). The percent of CD56+ NK cells that were CD107a+, that is, degranulated, was determined by flow cytometry after 1 hour of coincubation with T cells or positive control cells 721.221. (B,G) Data are from 3 independent experiments, and the total numbers of cells are indicated. (C-F,H) Representative data from 3 experiments are shown. *P <.05, **P <.005.
Next, 721.221 cells were treated for 18 hours with low concentrations of the microtubule-depolymerizing drug, colchicine, to increase the fraction of cells in mitosis from approximately 4% to approximately 75%. NK-cell degranulation, measured by NK-cell surface staining for CD107a (LAMP-1),9 was up to 4-fold greater in response to target cells arrested in mitosis compared with control-treated target cells (Figure 1C-D). A 1-hour treatment of target cells with a high concentration of colchicine, disrupting the target-cell cytoskeleton without arresting cells in mitosis, did not alter the NK-cell response (Figure 1C-D). Target cells arrested in mitosis using the drug nocodazole also triggered increased NK-cell degranulation (Figure 1E). To establish an assay independent of using drugs, the fraction of HT1080 fibrosarcoma cells in mitosis was enriched to about 40% to 50% by shaking off detached cells. NK-cell degranulation triggered by these cells was approximately twice that triggered by adherent HT1080 cells in other stages of the cell cycle (Figure 1F). Thus, susceptible target cells in mitosis trigger a heightened NK response compared to the same cells in other stages of the cell cycle.
We also found an increased binding of NK cells to lysis-resistant autologous T cells in mitosis (Figure 1G). However, NK-cell degranulation in response to autologous cells in mitosis remained low, even after 1 hour of coincubation (Figure 1H). Thus, mitosis triggers increased surveillance by NK cells but does not inevitably induce NK-cell responses; presumably, self-tolerance achieved through inhibitory NK-cell receptors remains dominant to allow healthy cells to undergo cell division.
We next set out to test for specific molecular recognition of cells in mitosis. Production of the pericellular matrix, including hyaluronan, increases to facilitate morphologic changes during mitosis.10 Treatment of 721.221 cells with hyaluronidase, which catalyzes degradation of hyaluronan, abrogated specific binding of cells in mitosis (Figure 2A). In contrast, increased binding of cells in mitosis was unaffected by degradation of other glycosaminoglycans (Figure 2A). Additionally, when NK cells were incubated with different glycosaminoglycans as putative blocking agents, only hyaluronan was able to prevent increased binding of NK cells to target cells in mitosis (Figure 2B). Thus, hyaluronan facilitates NK-cell recognition of cells in mitosis.
Evidence for molecular recognition of cells in mitosis by human NK cells. (A-H) The percent of 721.221 or Daudi cells in mitosis or other cell-cycle stages bound by NK cells after 10 minutes of coincubation, determined by confocal microscopy of cells stained for α-tubulin. (A) Target cells (721.221) were pretreated with different glycosaminoglycan-degrading enzymes: S hyalurolyticus hyaluronidase, which catalyzes degradation of hyaluronan; chondroitinase ABC, which degrades chondroitin sulfate; or heparinase I, which degrades heparin and to a lesser extent heparan sulfate. (B) NK cells were incubated with the different glycosaminoglycans indicated to test their ability to block specific recognition of target cells in mitosis. (C) NK cells were preincubated with an anti-CD44 mAb, which blocks the interaction between CD44 and hyaluronan or with a control isotype-matched anti-CD44 antibody that does not block this interaction. (D-E) NK cells were preincubated with monoclonal or polyclonal antibodies recognizing NKp46 or NKG2D, or isotype-matched control antibodies (i.c.), prior to coincubation with (D) 721.221 or (E) Daudi target cells. (F) NK-cell clones characterized by flow cytometry as having low (NKp46low; inset, dotted line) or high (NKp46high; inset, solid line) surface expression of NKp46 (inset: shaded histogram represents isotype-matched control staining) were compared for their capacity to bind 721.221 cells in mitosis or 721.221 in other stages of the cell cycle. (G) The binding of several NKp46high and NKp46low NK-cell clones to 721.221 cells is shown as the percentage increase in binding to cells in mitosis compared to cells in other stages of the cell cycle. (H) NK cells were preincubated with anti-NKp44 or control mAb. Data from at least 3 independent experiments are shown, and the total numbers of cells are indicated. *P <.05, **P <.005; ns indicates not significant.
Evidence for molecular recognition of cells in mitosis by human NK cells. (A-H) The percent of 721.221 or Daudi cells in mitosis or other cell-cycle stages bound by NK cells after 10 minutes of coincubation, determined by confocal microscopy of cells stained for α-tubulin. (A) Target cells (721.221) were pretreated with different glycosaminoglycan-degrading enzymes: S hyalurolyticus hyaluronidase, which catalyzes degradation of hyaluronan; chondroitinase ABC, which degrades chondroitin sulfate; or heparinase I, which degrades heparin and to a lesser extent heparan sulfate. (B) NK cells were incubated with the different glycosaminoglycans indicated to test their ability to block specific recognition of target cells in mitosis. (C) NK cells were preincubated with an anti-CD44 mAb, which blocks the interaction between CD44 and hyaluronan or with a control isotype-matched anti-CD44 antibody that does not block this interaction. (D-E) NK cells were preincubated with monoclonal or polyclonal antibodies recognizing NKp46 or NKG2D, or isotype-matched control antibodies (i.c.), prior to coincubation with (D) 721.221 or (E) Daudi target cells. (F) NK-cell clones characterized by flow cytometry as having low (NKp46low; inset, dotted line) or high (NKp46high; inset, solid line) surface expression of NKp46 (inset: shaded histogram represents isotype-matched control staining) were compared for their capacity to bind 721.221 cells in mitosis or 721.221 in other stages of the cell cycle. (G) The binding of several NKp46high and NKp46low NK-cell clones to 721.221 cells is shown as the percentage increase in binding to cells in mitosis compared to cells in other stages of the cell cycle. (H) NK cells were preincubated with anti-NKp44 or control mAb. Data from at least 3 independent experiments are shown, and the total numbers of cells are indicated. *P <.05, **P <.005; ns indicates not significant.
NK-cell CD44 was previously shown to bind hyaluronan on tumor cells and augment adhesion and cytotoxicity.11 However, increased NK-cell binding of cells in mitosis was unaffected by a CD44-blocking mAb (Figure 2C) used at a concentration sufficient to completely inhibit the high-level binding of hyaluronan to BW5147 cells (data not shown). Thus, receptors other than CD44 on NK cells are involved in recognition of cells in mitosis.
Natural cytotoxicity receptors (NCRs) are major activating NK-cell receptors with unknown cellular ligands.12 Mice lacking Ncr1 (NKp46 in humans) are impaired in their ability to clear certain tumors.13 Here, blocking NCR NKp46 with either monoclonal or polyclonal antibodies reduced NK-cell binding to 721.221 (Figure 2D) or Daudi (Figure 2E) cells in mitosis to the same level as cells in other cell-cycle stages. Blocking NKG2D, an activating NK-cell receptor not involved in lysis of 721.221,14 did not interfere with binding of cells in mitosis.
Additionally, NCR expression varies in NK cells,12 and NK-cell clones that expressed high levels of NKp46 showed more pronounced recognition of target cells in mitosis (Figure 2F-G). Antibody-mediated blocking of NKp46 on NKp46high NK cells reduced the binding of target cells in mitosis to a similar level as NKp46low NK cells (Figure 2F). Antibody-mediated blocking of the NCR NKp44, which is expressed on activated NK cells,15 also abrogated specific binding of 721.221 cells in mitosis (Figure 2H). That blocking either NKp46 or NKp44 was sufficient to abrogate increased binding to cells in mitosis is consistent with different NCR functioning synergistically.16,17 Thus, ligation of activating receptors involved in lysis of 721.221 facilitates increased binding of 721.221 cells in mitosis.
To test whether NKp46 directly binds hyaluronan, we used an enzyme-linked immunosorbent-type assay to determine if plate-bound hyaluronan bound immunoglobulin-fusion proteins of NCR or CD44 (positive control). Additionally, we tested if hyaluronan would stimulate reporter cells transfected to express a chimera consisting of the extracellular portion of NKp46 and the intracellular signaling domain of CD3 ζ, an approach used previously.18 Using these methodologies, we could not detect direct binding between NKp46 and hyaluronan (data not shown). Thus, although direct interaction between NCR and hyaluronan remains possible, alternatively, inside-out signaling via NCR could activate a hyaluronan-binding receptor19 or signaling via NCR and a hyaluronan-binding receptor could synergize to augment responses to cells in mitosis. Increased surveillance of cells in mitosis suggests an important novel strategy to safeguard tumor growth or viral replication.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: E.N.M.N.–'tH., C.R.A., H.Y., and D.M.D. designed research; E.N.M.N.–'tH., C.R.A, N.R.C., S.N., and H.Y. performed research; E.N.M.N.–'tH. analyzed data; and E.N.M.N.–'tH. and D.M.D. wrote the paper.
Acknowledgments
This work was supported by the Human Frontier Science Program, the Medical Research Council, Fundação para a Ciência e a Tecnologia, the Biotechnology and Biological Sciences Research Council, the Wellcome Trust, and a Lister Institute Research Prize.