Abstract
Accumulating evidence indicates that the cellular microenvironment plays a key role in follicular lymphoma (FL) pathogenesis, both within tumor lymph nodes (LNs) and in infiltrated bone marrow where ectopic LN-like reticular cells are integrated within malignant B-cell nodular aggregates. In normal secondary lymphoid organs, specific stromal cell subsets provide a highly specialized microenvironment that supports immune response. In particular, fibroblastic reticular cells (FRCs) mediate immune cell migration, adhesion, and reciprocal interactions. The role of FRCs and their postulated progenitors, that is, bone marrow mesenchymal stem cells (MSCs), in FL remains unexplored. In this study, we investigated the relationships between FRCs and MSCs and their capacity to sustain malignant B-cell growth. Our findings strongly suggest that secondary lymphoid organs contain MSCs able to give rise to adipocytes, chondrocytes, osteoblasts, as well as fully functional B-cell supportive FRCs. In vitro, bone marrow–derived MSCs acquire a complete FRC phenotype in response to a combination of tumor necrosis factor-α and lymphotoxin-α1β2. Moreover, MSCs recruit primary FL cells that, in turn, trigger their differentiation into FRCs, making them able to support malignant B-cell survival. Altogether, these new insights into the cross talk between lymphoma cells and their microenvironment could offer original therapeutic strategies.
Introduction
Several subsets of stromal cells, in particular follicular dendritic cells (FDCs) and fibroblastic reticular cells (FRCs), are found within secondary lymphoid organs where they play a key role in the initiation and maintenance of efficient immune responses. FDCs are restricted to germinal centers (GCs) and allow B-cell migration, selection, and differentiation through a complex set of survival factors including BCR-mediated signal, chemokines, cytokines, and adhesion molecules.1 B-cell selection relies on an affinity-based competition for the fixation of antigen, presented as immune complexes by CD21hiCD35hi FDCs. Only B cells with high-affinity BCR receive survival signals from FDCs, capture antigen, and present it to CD4+ T cells that deliver additional survival and maturation signals.2,3
Conversely, FRCs are less well characterized. They are tightly interconnected in the paracortex of lymph nodes (LNs) where they secrete and ensheath various extracellular matrix components, thus building an intricate network of conduits, connecting afferent lymphatic vessels to high endothelial venules (HEVs).4 This conduit system allows the rapid transport of soluble antigens from the periphery to the resident myeloid immature dendritic cells (DCs).5 In addition, part of this reticular network called cortical ridge favors B, T, and DC recruitment and reciprocal interactions, in particular through the production of constitutive and inflammatory chemokines.6,7 CCL19, CCL21, and CXCL12 are involved in the migration of mature myeloid DCs and naive B and T cells, whereas CXCL9, CXCL10, and CCL5 are crucial for the migration of activated T cells and plasmacytoid DCs.8-10 Strikingly, CCL19 and CCL21 are not synthesized by human HEVs but rather by stromal cells in the T-cell zone.11,12 These chemokines further reach luminal surface of HEVs by endothelial uptake and transcytosis. FRCs provide therefore a favorable and highly specialized lymphoid environment for immune cell migration and activation.
The ontogeny of FRCs and FDCs remains unclear, but these cells are supposed to be of mesenchymal origin. LN organogenesis in the mouse relies on the interaction between CD45−VCAM-1+ICAM-1+ mesenchymal cells and CD45+CD4+CD3− lymphoid tissue inducer cells in the LN anlagen.13 A prominent role is attributed to lymphotoxin-β receptor (LTβR) triggering by membrane-bound LTα1β2 (LT) and to tumor necrosis factor receptor 1 (TNFR1)/tumor necrosis factor-α (TNF).14,15 Adult lymphoid tissues are highly dynamic structures that retain several features of embryonic organization.16 Functional mouse FRCs able to construct a reticular meshwork and to secrete inflammatory chemokines could be generated in vitro by stimulation of LN-derived stromal cell lines using a combination of TNF and LT.17 In vivo, transgenic expression of LT or injection of newborn LN-derived LT+CD45+CD4+CD3− cells is sufficient to suppress mediate formation of ectopic LN-like structures including HEVs and FDCs.18-20 However, additional signals associated with an activation of the host immune system are required to get a complete organization and function of these tertiary lymphoid structures.19 Differentiation and maintenance of LN stromal cell subsets is thus strictly dependent on continual contact with activated lymphocytes. How FRCs and FDCs differentiate from a local or recruited mesenchymal progenitor remains unknown.
Follicular lymphomas (FLs) are the most frequent indolent non-Hodgkin lymphomas (NHLs) and result from the malignant transformation of GC-derived B cells.21 Microarray analyses have recently revealed that outcome for patients with FL is primarily predicted by specific molecular features of nonmalignant cells instead of tumor cells themselves.22 These results confirm FL dependency on a cross talk with their microenvironment, including CD4+ T cells, monocytes/macrophages, and stromal cells, that deliver growth factors required for lymphomagenesis. FDC and FRC networks are phenotypically and probably functionally altered during FL development. In particular, FL-infiltrating FDCs exhibit in most cases an undifferentiated phenotype,23 whereas FRC meshwork is up-regulated in this disease.24 However, cellular interactions between LN stromal cells and malignant B cells remain poorly understood.
Bone marrow (BM) involvement is found in up to 70% of FL cases at diagnosis.25 This infiltration is associated with the emergence of ectopic LN-like reticular cells, few of them expressing the FDC-associated markers CD21 and CD35.26 BM provides thus a preferred stromal microenvironment for FL cell growth. All normal BM stromal cell subsets derived from a common precursor, that is, the mesenchymal stem cell (MSC). MSCs are multipotent cells able to differentiate into several mesodermal lineages including adipocytes, chondrocytes, and osteoblasts.27 They have been found in adipose tissue, cartilage, and cord blood, but their preferential localization remains the BM. Whether or not LN-like stromal cells that develop in the BM following lymphoma cell infiltration arise from resident BM-MSCs has never been explored.
These observations support the hypothesis that at least 3 stromal cell subsets could be involved in the development of FL: FRCs and FDCs in lymphoid organs and MSCs in BM. In this study, we investigated in detail the phenotypic and functional links among these stromal compartments as well as their capacity to sustain malignant B-cell growth.
Materials and methods
Cell samples
Approval was obtained from the Rennes University Hospital Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. Stromal cells were obtained from human tonsils collected from children undergoing routine tonsillectomy, after informed consent was obtained. Tonsils were cut into pieces and flushed using syringe and needle. Cell suspension was treated with DNAse I (Pulmozyme, Roche, Neuilly sur Seine, France) and collagenase IV (Worthington, Freehold, NJ) followed by centrifugation on a discontinuous Percoll gradient (Amersham, Piscataway, NJ). LN-derived stromal cells called “Resto” were established from the 15%/25% Percoll interface by long-term culturing. Cells were initially allowed to adhere for 48 hours followed by elimination of nonadherent cells and culture in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% selected fetal calf serum (FCS; HyClone, Logan, UT), and penicillin/streptomycin (PS). Resto cells were used between passages 8 and 15. BM aspirates were collected from adult patients undergoing thoracic surgery, following informed consent. BM mononuclear cells were isolated by Ficoll density gradient and cultured (50 × 103 cells/cm2) in expansion medium containing α-MEM (Invitrogen), 10% FCS, and PS. After 2 days, nonadherent cells were discarded and adherent cells were replenished with fresh medium twice a week. When the culture reached confluency, BM-MSCs were detached and replated at 103 cells/cm2. MSCs were used from passages 2 to 5. Tonsil-derived MSCs (T-MSCs) were obtained from adherent tonsil cells depleted for CD3+, CD14+, CD16+, and CD19+ cells. The depleted fraction was cultured at 103 cells/cm2 in MSC expansion medium. Individual cell colonies were isolated and cells were further cloned at 0.3 cell/well in 96-well plates. Clonal T-MSCs that grew were expanded and tested for their adipogenic, osteogenic, chondrogenic, and FRC differentiation potential. Human foreskin fibroblasts (HFFs) were obtained from the American Type Culture Collection (ATCC; Rockville, MD).
B cells were purified from peripheral blood or FL LNs as the unbound fraction of magnetic cell sorting using the B-cell isolation kit II (Miltenyi Biotech, Bergisch Gladbach, Germany). Purity was more than 99% CD19+ B cells. In addition, more than 95% of B cells purified from FL samples expressed the appropriate κ or λ light chain according to the isotype of the tumor monoclonal immunoglobulin. The BL2 cell line was kindly provided by J. Wiels (IGR, Villejuif, France), whereas VAL and RL cell lines were a generous gift from C. Bastard (Centre Becquerel, Rouen, France).
Immature DCs (iDCs) were generated from peripheral blood monocytes isolated by elutriation on a Beckman JE-6B apparatus (Palo Alto, CA). Purified monocytes (>85% CD14+ cells) were cultured for 7 days at 2 × 106 cells/mL in RPMI 1640/10% FCS supplemented with 100 UI/mL granulocyte-macrophage colony-stimulating factor and 25 ng/mL IL-4 (both from AbCys, Paris, France). iDCs were large cells with an homogeneous HLA-DRhiCD40+CD80+CD86+CD83− phenotype.
Stromal cell stimulation
Stromal cells were stimulated by optimal doses of recombinant TNF-α (10 ng/mL) and LT-α1β2 (100 ng/mL; R&D Systems, Abingdon, United Kingdom) either for 3 days, for gene expression analysis and cell migration, or for 7 days, for adhesion and cell growth assays. For the induction of reticular meshwork, stromal cells were treated during 7 days by TNF or LT or both, or by coculture with normal B cells, primary FL B cells (7.5 × 105 cells/mL) or BL2 cell line (104 cells/mL).
Flow cytometry and immunofluorescence
Stromal cells were analyzed by flow cytometry using phycoerythrin (PE)–conjugated monoclonal antibody (mAb) to TNFR1, CD54 (Beckman Coulter, Villepinte, France), LTβR, CXCR4 (Becton Dickinson, San Diego, CA), CXCR3, CXCR5, CCR5, CCR1, CCR7 (R&D Systems), and CD106 (Chemicon, Temecula, CA). Isotype-matched mouse mAbs were used as negative control. Cells were analyzed using a FACSCalibur flow cytometer and CellQuest Pro Software (Becton Dickinson).
Resto cells, T-MSCs, BM-MSCs, and HFFs were grown on chamber slides with or without stimulation. Slides were fixed in cold acetone or in 4% paraformaldehyde-PBS. Cells were stained with transglutaminase (Abcam, Cambridge, United Kingdom), fibronectin (Chemicon), or isotype-matched control mAbs, followed by labeling with specific secondary antibodies conjugated to Fluoprobes 546 (Interchim, Montlucon, France). Coverslips were mounted with Vectashield mounting medium including DAPI (Vector Laboratories, Burlingame, CA) and examined using a deconvolution fluorescence microscope (DMRXA2, Leica, Wetzlar, Germany) equipped with a 40 ×/1.00 numerical aperture (NA) oil objective. Digital images were acquired using a CoolSnap ES charge-coupled device (CCD) camera (Roper Scientific, Trenton, NJ) and were processed using Metamorph software (Molecular Devices, Downington, PA).
Quantitative RT-PCR
RNA was extracted using RNeasy Kit (Qiagen, Valencia, CA) and cDNA was generated using Superscript II reverse transcriptase (Invitrogen). For quantitative reverse transcription–polymerase chain reaction (RT-PCR), we used assay-on-demand primers and probes, and the TaqMan Universal Master Mix from Applied Biosystems (Foster City, CA). Gene expression was measured using the ABI Prism 7700 Sequence Detection System. ABL was determined as the appropriate internal standard gene. For each sample, the CT value for the gene of interest was determined, normalized to its respective value for ABL, and compared to the value obtained for unstimulated cells.
Adhesion assays
Tonsil cells or iDCs were induced to adhere to a TNF/LT-pretreated or unstimulated confluent stromal monolayer for 2 hours. After the unbound cells were removed by washing 4 times with PBS, adherent cells were collected and stained with FITC-conjugated anti-CD45 mAb. Data were expressed as the ratio of CD45+ bound cells to CD45− stromal cells. The same experiment was done using unpurified FL samples. Adhesion of tumor B cells was evaluated using FITC-conjugated anti-CD19 and PE-conjugated anti-CD45 mAbs as the ratio of CD19+ bound FL B cells to CD45− stromal cells.
Migration assay
BL2 cell line or primary unpurified FL cells stained with FITC-conjugated anti-CD19 mAb were added at 105 cells/100 μL to the upper compartment of Transwell chambers with 5-μm pore filters (Costar, Cambridge, MA). Lower chambers contained BM-MSC supernatants or RPMI/10% FCS supplemented or not with CXCL12α (100 ng/mL; R&D Systems). In some experiments, BL2 cells were preincubated with 350 ng/mL AMD3100 (Sigma, St Louis, MO) for 30 minutes at 37°C. Migrated cells were collected after 5 hours and the absolute number of viable CD19+TOPRO-3− FL cells, or TOPRO-3− BL2 cells was evaluated by flow cytometry using FlowCount beads (Beckman Coulter). Data are expressed as a migration index corresponding to the number of cells migrating in response to tested medium divided by the number of cells migrating in response to control medium.
B-cell death
Tumor B cells were cultured alone or on a confluent stromal cell layer pretreated or not with TNF/LT. B-cell death was always analyzed on selectively gated CD45+ B cells using FITC-conjugated anti-CD45 mAb (Beckman Coulter). For BL2 cell line, serum deprivation–induced apoptosis was analyzed on day 3 using active caspase 3 PE apoptosis kit (Becton Dickinson) according to manufacturer's instructions. For primary FL samples, purified CD19+ cells were maintained in RPMI 1640/10%FCS and spontaneous cell death was evaluated on day 5 by staining with TOPRO-3 (Invitrogen). The absolute number of viable CD45+TOPRO-3− FL cells was evaluated using FlowCount beads.
B-cell proliferation assay
Confluent stromal cells pretreated or not with TNF/LT in 96-well plates were washed before the addition of BL2 (5 × 103 cells/well), VAL, or RL (103 cells/well) in RPMI 1640 with low serum concentration. After 3 days of culture, cells were pulsed with 1 μCi/well (0.037 MBq) tritiated thymidine (3H-TdR, Amersham) for the last 12 hours of culture, harvested, and counted on a liquid scintillation analyser.
In vitro differentiation of T-MSCs
For adipogenic differentiation, confluent T-MSCs were grown in expansion medium supplemented with 1 μM dexamethasone (Vianex SA, Attica, Greece), 0.35 mM hydrocortisone (Pharmacia, Guyancourt, France), 100 μg/mL 3-isobutyl-1-methylxanthine, 0.1 μg/mL insulin, and 60 μM indomethacin (Sigma). Cultures were maintained for 2 weeks before fixation and staining with Oil-red O (Sigma). Osteoblast differentiation was achieved after a 3-week culture of T-MSCs in expansion medium supplemented with 10 μM dexamethasone, 0.1 mM ascorbic acid, and 10 mM β-glycerophosphate (Sigma). Cells were then washed, fixed, and stained with Alizarin red (Sigma). To stimulate chondrogenic differentiation, 2.5 × 105 T-MSCs were pelleted and maintained for 4 weeks using the hMSC chondrogenic differentiation medium (Cambrex Biosciences, Walkersville, MD) plus 10 ng/mL TGF-β3 (R&D Systems). Pellets were then formalin-fixed, frozen, and cryostat sections were stained with Alcian blue (Sigma).
Statistical analyses
Statistical analyses were performed with the nonparametric Wilcoxon test or the Student t test for pairs.
Results
Human tonsil–derived stromal cells exhibit FRC features
Studies on human LN stromal cells have been hampered by the difficulty of purifying them and by their high heterogeneity. We thus decided to establish and characterize new human LN–derived stromal cells. We derived 15 primary stromal cell cultures from human tonsils that we named Resto. Resto cells showed typical spindle-shaped fibroblastic morphology. They did not express the short B cell–specific CD21 isoform, the long FDC-specific CD21 isoform, or CD45. However, they stained positively for mesenchymal markers including CD90, CD73, and CD105 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
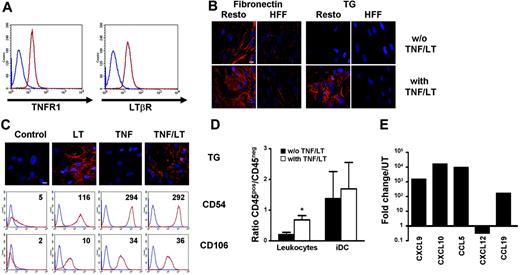
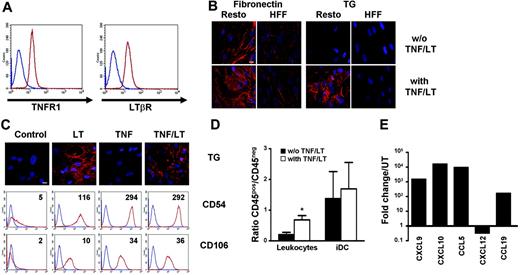
FRCs are essentially defined by their functional properties. In particular, they construct reticular meshwork and secrete chemokines in response to TNF/LT. At first, we showed that Resto cells uniformly expressed TNFR1 and LTβR (Figure 1A). Extracellular matrix organization was studied using staining for fibronectin and transglutaminase, which are strongly secreted in vivo and in vitro by human FRCs.17,24 In the absence of stimulation, transglutaminase could not be detected on the cell surface, whereas fibronectin staining was weak with an oriented pattern showing aligned fibers. After 7 days of treatment by a combination of TNF and LT, transglutaminase and fibronectin were redistributed with a similar pattern, that is, most of the reactivity revealed along interconnected fibrillar extracellular structures (Figure 1B). This meshwork was observed without cell permeabilization, indicating that it was truly secreted outside the cells. In comparison, HFFs cultured under the same conditions did not produce such extracellular reticular network even though they spontaneously secreted low amounts of oriented fibronectin-containing fibers. Of note, neither transglutaminase nor fibronectin mRNA levels, evaluated by quantitative RT-PCR, were enhanced after 12 hours, 3 days, or 7 days of Resto cell culture in the presence of TNF/LT (not shown), indicating that the modification of transglutaminase and fibronectin distribution was unlikely due to up-regulation of transcription but rather to protein relocalization. Interestingly, TNF alone was able to induce a strong and homogeneous increase in CD54/ICAM-1 and CD106/VCAM-1 expression but not the transglutaminase meshwork formation (Figure 1C). In contrast, LT was less efficient in enhancing the expression of adhesion molecules but induced a dense meshwork that was not significantly more developed when TNF was added to LT.
Modulation of Resto properties under TNF/LT stimulation. (A) Membrane expression of TNFR1 and LTβR on Resto cells. Blue lines indicate immunoglobulin control, red lines indicate specific staining. (B) Induction of an extracellular meshwork. Resto cells and HFFs were cultured for 7 days with or without TNF/LT stimulation. Microscopic visualization of a dense meshwork of extracellular matrix fibers was performed with fibronectin and transglutaminase (TG) extracellular staining. Bar represents 20 μm. (C) Differential effects of TNF and LT treatment. Resto cells were cultured with TNF, LT, TNF/LT or without stimulation for 7 days before transglutaminase (TG), CD54, and CD106 staining. TG was detected by microscopic analysis. Bar represents 20 μm. CD54 and CD106 were revealed by flow cytometry. Blue lines indicate immunoglobulin control, red lines indicate specific staining. Ratio of mean fluorescence intensity (mean fluorescence intensity of CD54 or CD106/mean intensity fluorescence of immunoglobulin-specific control) is indicated on the top right of each panel. (D) Adhesion assay. Tonsil leukocytes or monocyte-derived immature DCs (iDCs) were incubated for 2 hours with Resto cells pretreated or not with TNF/LT for 7 days. Unbound cells were then removed and adherent CD45+ cells and stromal CD45− cells were collected using trypsin and quantified by flow cytometry. Results are the mean values of the ratio of CD45+ to CD45− cells (n = 4). The error bars indicate the SD about the mean. *Mean value is statistically different from that of coculture with unstimulated Resto cells (P < .01). (E) Real-time PCR quantification of chemokine expression. Resto cells were cultured for 3 days with TNF/LT and were then analyzed for CXCL9, CXCL10, CCL5, CXCL12, and CCL19 expression. Each sample was normalized to ABL and compared with expression levels in untreated (UT) Resto cells. The arbitrary value of 1 was assigned to UT Resto cells. CCL19 was not detected in UT Resto cells and the ΔΔCT was then calculated with an arbitrary CT value of 40. Results are those of 1 experiment of 3.
Modulation of Resto properties under TNF/LT stimulation. (A) Membrane expression of TNFR1 and LTβR on Resto cells. Blue lines indicate immunoglobulin control, red lines indicate specific staining. (B) Induction of an extracellular meshwork. Resto cells and HFFs were cultured for 7 days with or without TNF/LT stimulation. Microscopic visualization of a dense meshwork of extracellular matrix fibers was performed with fibronectin and transglutaminase (TG) extracellular staining. Bar represents 20 μm. (C) Differential effects of TNF and LT treatment. Resto cells were cultured with TNF, LT, TNF/LT or without stimulation for 7 days before transglutaminase (TG), CD54, and CD106 staining. TG was detected by microscopic analysis. Bar represents 20 μm. CD54 and CD106 were revealed by flow cytometry. Blue lines indicate immunoglobulin control, red lines indicate specific staining. Ratio of mean fluorescence intensity (mean fluorescence intensity of CD54 or CD106/mean intensity fluorescence of immunoglobulin-specific control) is indicated on the top right of each panel. (D) Adhesion assay. Tonsil leukocytes or monocyte-derived immature DCs (iDCs) were incubated for 2 hours with Resto cells pretreated or not with TNF/LT for 7 days. Unbound cells were then removed and adherent CD45+ cells and stromal CD45− cells were collected using trypsin and quantified by flow cytometry. Results are the mean values of the ratio of CD45+ to CD45− cells (n = 4). The error bars indicate the SD about the mean. *Mean value is statistically different from that of coculture with unstimulated Resto cells (P < .01). (E) Real-time PCR quantification of chemokine expression. Resto cells were cultured for 3 days with TNF/LT and were then analyzed for CXCL9, CXCL10, CCL5, CXCL12, and CCL19 expression. Each sample was normalized to ABL and compared with expression levels in untreated (UT) Resto cells. The arbitrary value of 1 was assigned to UT Resto cells. CCL19 was not detected in UT Resto cells and the ΔΔCT was then calculated with an arbitrary CT value of 40. Results are those of 1 experiment of 3.
In agreement with the induction of adhesion molecules and extracellular matrix reorganization, TNF/LT pretreatment significantly improved the adhesion of CD45+ tonsil leukocytes on Resto cells (Figure 1D, P < .01). Interestingly, the binding of a pure population of myeloid iDCs was strong on unstimulated Resto cells and was not increased after TNF/LT stimulation. In addition, even if Resto cells spontaneously expressed inflammatory chemokines CXCL9, CXCL10, and CCL5, their levels of expression were strongly enhanced by TNF/LT (mean value 586-fold, 7498-fold, and 3783-fold, respectively, n = 3, Figure 1E). In contrast, CXCL12 was slightly down-regulated under the same culture conditions even if it remained strongly expressed. CCL19 mRNA was undetectable in the steady state but was induced in response to TNF/LT. Finally, the regulation of chemokine expression was essentially mediated by TNF, whereas LT was less potent (not shown).
Collectively, these data strongly suggest that Resto cells have all the morphologic, phenotypic, and functional features of FRCs and are suitable for in vitro studies of interactions with B cells. In addition, the combination of TNF and LT is required to simultaneously induce adhesion molecules, inflammatory and LN-specific chemokines, and organization of a dense reticular meshwork.
Tonsil-derived FRCs support tumor B-cell growth
We tested the capacity of Resto cells to promote the growth of 3 widely used GC-derived B-cell lines: BL2, VAL, and RL. These 3 cell lines were cultured in a low concentration of serum that did not allow their autonomous growth (tritiated thymidine incorporation <500 cpm after 3 days of culture). Untreated Resto cells significantly enhanced lymphoma B-cell growth (P<.05) through a strong inhibition of serum deprivation–induced apoptosis (Figure 2A-B). Moreover, this effect was significantly reinforced by preliminary treatment of Resto cells with TNF/LT (P<.05) due to an additional B-cell survival improvement. As an example, BL2 apoptosis, assessed by active caspase-3 staining, was 80.7% ± 10.4% in medium alone, 26.5% ± 17.9% in coculture with untreated Resto cells, and 1.6% ± 0.7% in coculture with conditioned Resto cells (P<.05 between the 3 groups; n = 5).
Resto cells display a tumor B-cell supportive effect. (A) Growth of tumor B cell lines. BL2, VAL, and RL cells lines were cultured for 3 days in low serum concentration alone, or with confluent Resto cells, pretreated or not with TNF/LT for 7 days. Proliferation was assessed by tritiated thymidine (3H-TdR) incorporation determined in sixplicate culture wells. Stromal cells cultured alone always showed tritiated thymidine incorporation less than 500 cpm. Data are expressed as percentages of thymidine incorporation by B-cell lines cultured in the presence of Resto cells (pretreated or not with TNF/LT) with respect to B-cell lines alone (assigned to 100%). Bars represent mean values ± SD from 4 (BL2) or 5 (VAL, RL) independent experiments. *Mean value is statistically significantly different from that obtained without stromal cells (P<.05). **Mean value is statistically significantly different from that obtained with untreated Resto cells (P<.05). (B) Apoptosis of tumor B cell lines. BL2 cells were cultured under low serum concentration alone or on confluent Resto cells pretreated or not with TNF/LT. Cells were recovered after 3 days of coculture and CD45+ apoptotic cells were detected by staining with active caspase-3 staining. Percentage of caspase-3+ cells is indicated in each panel. Results are those of 1 experiment representative of 5.
Resto cells display a tumor B-cell supportive effect. (A) Growth of tumor B cell lines. BL2, VAL, and RL cells lines were cultured for 3 days in low serum concentration alone, or with confluent Resto cells, pretreated or not with TNF/LT for 7 days. Proliferation was assessed by tritiated thymidine (3H-TdR) incorporation determined in sixplicate culture wells. Stromal cells cultured alone always showed tritiated thymidine incorporation less than 500 cpm. Data are expressed as percentages of thymidine incorporation by B-cell lines cultured in the presence of Resto cells (pretreated or not with TNF/LT) with respect to B-cell lines alone (assigned to 100%). Bars represent mean values ± SD from 4 (BL2) or 5 (VAL, RL) independent experiments. *Mean value is statistically significantly different from that obtained without stromal cells (P<.05). **Mean value is statistically significantly different from that obtained with untreated Resto cells (P<.05). (B) Apoptosis of tumor B cell lines. BL2 cells were cultured under low serum concentration alone or on confluent Resto cells pretreated or not with TNF/LT. Cells were recovered after 3 days of coculture and CD45+ apoptotic cells were detected by staining with active caspase-3 staining. Percentage of caspase-3+ cells is indicated in each panel. Results are those of 1 experiment representative of 5.
Taken together, our results show that tonsil-derived FRC-like cells are able to support the growth of human GC–derived lymphoma B-cell lines.
Tonsils contain MSC-like progenitors that could acquire an FRC phenotype
Because LN stromal cells are supposed to be of mesenchymal origin, we looked for the presence of a tonsil mesenchymal precursor able to give rise to FRCs.
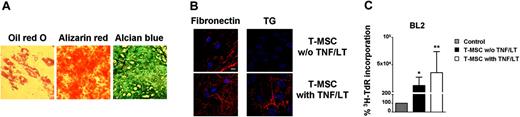
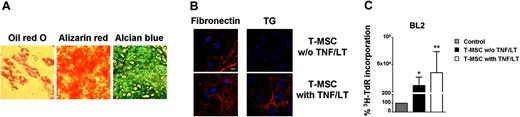
When lineage-negative tonsil cells were cultured in conditions close to these initially described for BM-MSCs, adherent colonies of cells morphologically resembling fibroblasts (CFU-Fs) were obtained between day 10 and 14. Limiting dilution assay was additionally performed to evaluate the presence of multipotent mesenchymal progenitors in secondary lymphoid organs. Phenotypically, these clonally expanded cells expressed all markers of MSC (CD90+CD73+CD105+CD45−, not shown). To unequivocally identify them as T-MSCs we tested their potential of differentiation. Monolayer cultures treated with adipogenic supplements showed cytoplasmic lipid droplets visualized by staining with Oil-red O. After culture under osteogenic conditions, cells broadened and formed mineralized matrix as evidenced by Alizarin red staining. Finally, high-density pellet cell cultures maintained in chondrogenic medium were composed of chondrocyte-like cells surrounded by a sulfated proteoglycan-rich Alcian blue–positive extracellular matrix (Figure 3A). Gene expression analysis by quantitative RT-PCR showed a strong up-regulation of lipoprotein lipase, osteopontin, and aggrecan during adipogenic, osteogenic, and chondrogenic differentiation, respectively (not shown). Thus, tonsils contain fibroblast-like cells that can be clonally expanded in vitro while retaining the capacity to differentiate into at least 3 mesodermal lineages and could therefore be considered bona fide T-MSCs. Interestingly, resident T-MSCs could also acquire characteristics of FRCs after treatment with TNF/LT, including the construction of a latticelike network structure (Figure 3B) and the capacity to support tumor B-cell growth (Figure 3C).
Tonsils contain pluripotent MSCs. (A) Differentiation of T-MSCs. After a 14- to 28-day culture in appropriate differentiation media, T-MSCs were stained with Oil-red O (left), Alizarin red (middle), and Alcian blue (right) to reveal the presence of adipocyte-specific lipid vacuoles, osteoblast-specific calcium deposits, and chondrocyte-specific proteoglycans, respectively. Cells were examined using an Olympus IX71 inverted microscope (Olympus, Tokyo, Japan) equipped with a 40 ×/0.60 NA objective, and digital images were acquired using an Olympus Camedia C-5060 camera. (B) Induction of an extracellular meshwork. T-MSCs were stained for fibronectin and transglutaminase (TG) after 7 days of culture with or without stimulation with TNF/LT. Bar represents 20 μm. (C) Growth of BL2 cell line. BL2 was cultured for 3 days in low serum concentration alone, or with confluent clonal T-MSC, pretreated or not with TNF/LT for 7 days. Proliferation was assessed by tritiated thymidine (3H-TdR) incorporation determined in sixplicate culture wells. Stromal cells cultured alone always showed tritiated thymidine incorporation less than 500 cpm. Data are expressed as percentages of thymidine incorporation by BL2 cells cultured in the presence of clonal T-MSCs (pretreated or not with TNF/LT) with respect to BL2 cells alone (assigned to 100%). Bars represent mean values ± SD from 6 independent experiments. *Mean value is statistically significantly different from that obtained without stromal cells (P<.05). **Mean value is statistically significantly different from that obtained with untreated T-MSC (P<.05).
Tonsils contain pluripotent MSCs. (A) Differentiation of T-MSCs. After a 14- to 28-day culture in appropriate differentiation media, T-MSCs were stained with Oil-red O (left), Alizarin red (middle), and Alcian blue (right) to reveal the presence of adipocyte-specific lipid vacuoles, osteoblast-specific calcium deposits, and chondrocyte-specific proteoglycans, respectively. Cells were examined using an Olympus IX71 inverted microscope (Olympus, Tokyo, Japan) equipped with a 40 ×/0.60 NA objective, and digital images were acquired using an Olympus Camedia C-5060 camera. (B) Induction of an extracellular meshwork. T-MSCs were stained for fibronectin and transglutaminase (TG) after 7 days of culture with or without stimulation with TNF/LT. Bar represents 20 μm. (C) Growth of BL2 cell line. BL2 was cultured for 3 days in low serum concentration alone, or with confluent clonal T-MSC, pretreated or not with TNF/LT for 7 days. Proliferation was assessed by tritiated thymidine (3H-TdR) incorporation determined in sixplicate culture wells. Stromal cells cultured alone always showed tritiated thymidine incorporation less than 500 cpm. Data are expressed as percentages of thymidine incorporation by BL2 cells cultured in the presence of clonal T-MSCs (pretreated or not with TNF/LT) with respect to BL2 cells alone (assigned to 100%). Bars represent mean values ± SD from 6 independent experiments. *Mean value is statistically significantly different from that obtained without stromal cells (P<.05). **Mean value is statistically significantly different from that obtained with untreated T-MSC (P<.05).
BM-MSCs could differentiate into FRCs
BM is the main reservoir of MSCs. BM-MSCs are thought to migrate to various organs in the context of tissue remodeling and represent a source of pluripotent cells for the repair of damaged tissues.28,29 Their capacity to migrate into secondary lymphoid organs and generate LN-specific stromal cells has never been explored. We examined therefore whether BM-MSCs could differentiate into LN-like FRCs.
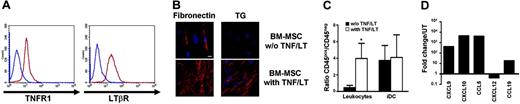
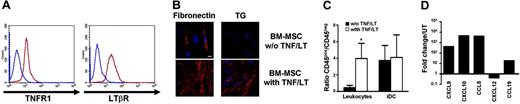
Starting from adherent BM mononuclear cells, we reproducibly obtained BM-MSCs able to differentiate into osteogenic, adipogenic, and chondrogenic lineages using appropriate inducer media (not shown). Expression of TNFR1 and LTβR on these cells was very similar to that found on Resto cells (Figure 4A). Treatment of BM-MSCs with TNF/LT during 7 days resulted in the up-regulation of CD54 and CD106 expression as well as the gathering of a dense extracellular reticular meshwork positive for transglutaminase and fibronectin staining (Figure 4B). Concomitantly, adhesion of tonsil leukocytes was markedly enhanced (P<.05), whereas iDCs were already firmly adherent on untreated BM-MSCs (Figure 4C). Finally, similarly to Resto cells, BM-MSCs produced high levels of CXCL9, CXCL10, and CCL5 and slightly down-regulated CXCL12 expression in response to TNF/LT stimulation. Moreover, the LN-specific chemokine CCL19 was also induced in BM-MSCs (Figure 4D).
BM-MSCs acquire complete functional characteristics of FRCs under TNF/LT stimulation. (A) Membrane expression of TNFR1 and LTβR on BM-MSCs. Blue lines indicate immunoglobulin control, red lines indicate specific staining. (B) Meshwork induction. BM-MSCs were cultured with or without TNF/LT stimulation for 7 days. Microscopic visualization of a meshwork of extracellular matrix fibers was performed with fibronectin and transglutaminase (TG) staining. Bar represents 20 μm. (C) Adhesion assay. Tonsil leukocytes or monocyte-derived iDCs were incubated for 2 hours with BM-MSCs pretreated or not with TNF/LT for 7 days. Unbound cells were then removed and adherent CD45+ cells and stromal CD45− cells were collected using trypsin and quantified by flow cytometry. Results are the mean values of the ratio of CD45+ to CD45− cells (n = 4). The error bars indicate the SD of the mean. *Mean value is statistically different from that of coculture with unstimulated BM-MSCs (P<.05). (D) Real-time PCR quantification of chemokine expression. BM-MSCs were cultured for 3 days with TNF/LT and were then analyzed for CXCL9, CXCL10, CCL5, CXCL12, and CCL19 expression. Each sample was normalized to ABL and compared to expression levels in untreated (UT) BM-MSCs. The arbitrary value of 1 was assigned to UT BM-MSCs. CCL19 was not detected in UT Resto cells and the ΔΔCT was then calculated with an arbitrary CT value of 40. Results are those of one experiment of 3.
BM-MSCs acquire complete functional characteristics of FRCs under TNF/LT stimulation. (A) Membrane expression of TNFR1 and LTβR on BM-MSCs. Blue lines indicate immunoglobulin control, red lines indicate specific staining. (B) Meshwork induction. BM-MSCs were cultured with or without TNF/LT stimulation for 7 days. Microscopic visualization of a meshwork of extracellular matrix fibers was performed with fibronectin and transglutaminase (TG) staining. Bar represents 20 μm. (C) Adhesion assay. Tonsil leukocytes or monocyte-derived iDCs were incubated for 2 hours with BM-MSCs pretreated or not with TNF/LT for 7 days. Unbound cells were then removed and adherent CD45+ cells and stromal CD45− cells were collected using trypsin and quantified by flow cytometry. Results are the mean values of the ratio of CD45+ to CD45− cells (n = 4). The error bars indicate the SD of the mean. *Mean value is statistically different from that of coculture with unstimulated BM-MSCs (P<.05). (D) Real-time PCR quantification of chemokine expression. BM-MSCs were cultured for 3 days with TNF/LT and were then analyzed for CXCL9, CXCL10, CCL5, CXCL12, and CCL19 expression. Each sample was normalized to ABL and compared to expression levels in untreated (UT) BM-MSCs. The arbitrary value of 1 was assigned to UT BM-MSCs. CCL19 was not detected in UT Resto cells and the ΔΔCT was then calculated with an arbitrary CT value of 40. Results are those of one experiment of 3.
In conclusion, BM-MSCs could acquire in vitro, in response to TNF/LT, a complete FRC phenotype not distinguishable from that obtained using LN-derived stromal cell lines.
Human lymphoma B cells induced the differentiation of BM-MSCs into FRC-like supportive cells
Given the frequent medullar involvement in FL and its association with the development of LN-like stromal cells, an attractive hypothesis was that BM-MSCs could differentiate into functional FRCs in situ in response to stimulation by tumor B cells.
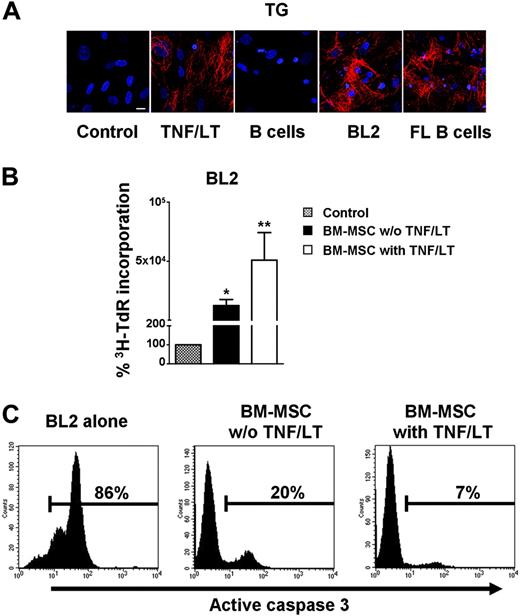
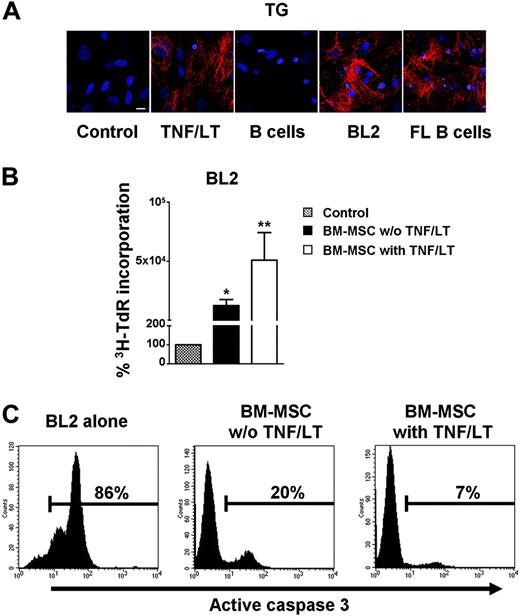
To explore this possibility, confluent BM-MSCs were cultured for 7 days in the presence of BL2 cell line or purified CD19+ primary FL cells (Figure 5A). Tumor B cells induced the formation of a full filamentous meshwork comprising transglutaminase fibers. This meshwork had a pattern very similar to that obtained following treatment with TNF/LT. In contrast, normal B cells purified from peripheral blood could not induce such intricate extracellular matrix reorganization. Lymphoma B cells, unlike normal B cells, promote thus the differentiation of BM-MSCs into cells with FRC features. We next sought to determine whether these BM-MSC–derived FRC-like cells became fully competent as tumor B-cell feeders. As shown in Figure 5B, BM-MSCs strongly reversed BL2 growth arrest induced by serum deprivation. Of interest, this growth-promoting effect was significantly enhanced when BM-MSCs were conditioned with TNF/LT (P<.05; n = 4). Untreated BM-MSCs had a strong, although not complete, protective effect on serum deprivation–induced apoptosis (mean percentage of CD45+caspase-3+ cells: 24.8% ± 17.5%, n = 5) and pretreatment with TNF/LT fully restored BL2 viability (mean percentage of CD45+caspase-3+ cells: 7.4% ± 4.7%, n = 5; Figure 5C).
Bidirectional interaction between tumor B cells and BM-MSCs. (A) Meshwork induction. BM-MSCs were cultured alone or in the presence of TNF/LT, purified CD19+ peripheral blood B cells, BL2 cell line, or purified CD19+ primary FL B cells for 7 days. Expression of transglutaminase (TG) was revealed by fluorescence microscopy. Bar represents 20 μm. (B) Growth of tumor B cell lines. BL2 was cocultured for 3 days in low serum concentration alone or with confluent BM-MSCs, pretreated or not with TNF/LT for 7 days. Cell growth was assessed by tritiated thymidine (3H-TdR) incorporation determined in sixplicate culture wells. Stromal cells cultured alone always showed tritiated thymidine incorporation less than 500 cpm. Data are expressed as percentages of the thymidine incorporation by BL2 cells cultured in the presence of BM-MSCs (pretreated or not with TNF/LT) with respect to BL2 cells alone (assigned to 100%). Results represent mean values ± SD from 4 independent experiments. *Mean value is statistically significantly different from that obtained without stromal cells (P<.05). **Mean value is statistically significantly different from that obtained with untreated BM-MSCs (P<.05). (C) Apoptosis of BL2 cell line. Confluent BM-MSCs pretreated or not with TNF/LT for 7 days were thereafter cocultured with BL2 cell line under limiting serum concentration. Apoptotic CD45+ tumor cells were detected by active caspase-3 staining after 3 days of coculture. Percentage of caspase-3+ cells is indicated in each panel. Results are those of 1 experiment representative of 5.
Bidirectional interaction between tumor B cells and BM-MSCs. (A) Meshwork induction. BM-MSCs were cultured alone or in the presence of TNF/LT, purified CD19+ peripheral blood B cells, BL2 cell line, or purified CD19+ primary FL B cells for 7 days. Expression of transglutaminase (TG) was revealed by fluorescence microscopy. Bar represents 20 μm. (B) Growth of tumor B cell lines. BL2 was cocultured for 3 days in low serum concentration alone or with confluent BM-MSCs, pretreated or not with TNF/LT for 7 days. Cell growth was assessed by tritiated thymidine (3H-TdR) incorporation determined in sixplicate culture wells. Stromal cells cultured alone always showed tritiated thymidine incorporation less than 500 cpm. Data are expressed as percentages of the thymidine incorporation by BL2 cells cultured in the presence of BM-MSCs (pretreated or not with TNF/LT) with respect to BL2 cells alone (assigned to 100%). Results represent mean values ± SD from 4 independent experiments. *Mean value is statistically significantly different from that obtained without stromal cells (P<.05). **Mean value is statistically significantly different from that obtained with untreated BM-MSCs (P<.05). (C) Apoptosis of BL2 cell line. Confluent BM-MSCs pretreated or not with TNF/LT for 7 days were thereafter cocultured with BL2 cell line under limiting serum concentration. Apoptotic CD45+ tumor cells were detected by active caspase-3 staining after 3 days of coculture. Percentage of caspase-3+ cells is indicated in each panel. Results are those of 1 experiment representative of 5.
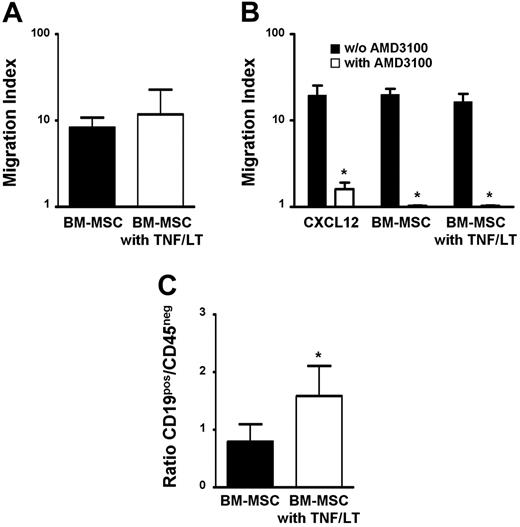
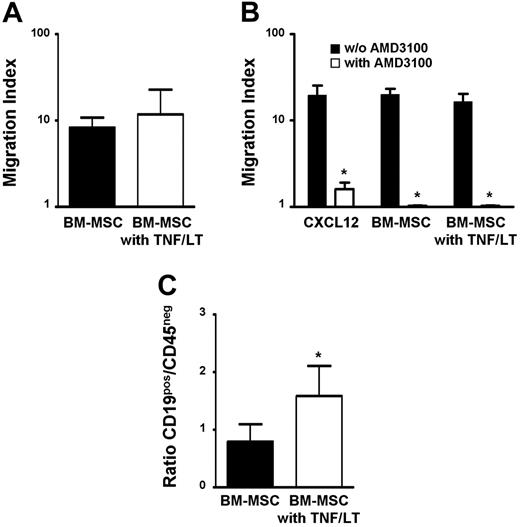
To locally promote this conversion of BM-MSCs into FRC-like cells, FL cells must be specifically recruited by resident MSCs into the medullary compartment. BM-MSC supernatant efficiently induced chemotaxis of primary FL cells, which was not enhanced by pretreatment of stromal cells with TNF/LT (Figure 6A). CD19+ FL cells and BL2 cell line shared a common pattern of chemokine receptor expression, that is, CXCR4hiCXCR5hiCCR7loCCR5loCXCR3−CCR1− (data not shown).30-33 Among the chemokines produced by stromal cells in our culture system, CXCL12 was the best candidate as malignant B-cell chemoattractant. In particular, it was highly expressed by untreated BM-MSCs and was not induced by TNF/LT stimulation (Figure 4D). In agreement, BL2 migrated efficiently toward CXCL12 and this migration was specifically abrogated by AMD3100, the selective antagonist of CXCR4. Moreover, addition of AMD3100 to BM-MSC supernatants completely blocked BL2 migration, independently of TNF/LT stimulation (Figure 6B). These data suggest that CXCL12 is the central chemokine involved in the migration of GC-derived malignant B cells toward BM-MSCs.
BM-MSCs mediate malignant B-cell migration and adhesion. (A) Migration assay. Primary LN samples from patients with FL were stained with anti-CD19 mAb and subjected to the chemotaxis assay for migration in response to supernatants from BM-MSCs treated or not with TNF/LT for 3 days. Migration index is calculated as the number of viable CD19+TOPRO-3− FL B cells migrating in response to the BM-MSC supernatant divided by the number of viable CD19+TOPRO-3− FL B cells migrating in response to culture medium. Results are the mean ± SD from 3 FL patients. (B) Role of CXCL12 in malignant B-cell migration. BL2 cell line was subjected to the chemotaxis assay in response to CXCL12 or to supernatants from BM-MSCs treated or not with TNF/LT for 3 days. When indicated, BL2 was preincubated with the CXCR4 antagonist AMD3100. Results are expressed as the migration index corresponding to the number of cells migrating in response to the chemokine or to the BM-MSC supernatant divided by the number of cells migrating in response to appropriate control medium. Results represent the mean values ± SD from 3 independent experiments. *Mean value is statistically significantly different from that obtained without AMD3100 (P<.05). (C) Adhesion assay. Primary unpurified FL samples were incubated for 2 hours with BM-MSCs pretreated or not with TNF/LT for 7 days. Unbound cells were then removed and adherent cells, including CD19+CD45+ malignant B cells, CD19−CD45+ nonmalignant hematopoietic cells, and CD45− stromal cells were collected using trypsin and quantified by flow cytometry. Results are the mean values of the ratio of CD19+ to CD45− cells (n = 4). The error bars indicate the SD of the mean. *Mean value is statistically different from that of coculture with unstimulated stromal cells (P<.05).
BM-MSCs mediate malignant B-cell migration and adhesion. (A) Migration assay. Primary LN samples from patients with FL were stained with anti-CD19 mAb and subjected to the chemotaxis assay for migration in response to supernatants from BM-MSCs treated or not with TNF/LT for 3 days. Migration index is calculated as the number of viable CD19+TOPRO-3− FL B cells migrating in response to the BM-MSC supernatant divided by the number of viable CD19+TOPRO-3− FL B cells migrating in response to culture medium. Results are the mean ± SD from 3 FL patients. (B) Role of CXCL12 in malignant B-cell migration. BL2 cell line was subjected to the chemotaxis assay in response to CXCL12 or to supernatants from BM-MSCs treated or not with TNF/LT for 3 days. When indicated, BL2 was preincubated with the CXCR4 antagonist AMD3100. Results are expressed as the migration index corresponding to the number of cells migrating in response to the chemokine or to the BM-MSC supernatant divided by the number of cells migrating in response to appropriate control medium. Results represent the mean values ± SD from 3 independent experiments. *Mean value is statistically significantly different from that obtained without AMD3100 (P<.05). (C) Adhesion assay. Primary unpurified FL samples were incubated for 2 hours with BM-MSCs pretreated or not with TNF/LT for 7 days. Unbound cells were then removed and adherent cells, including CD19+CD45+ malignant B cells, CD19−CD45+ nonmalignant hematopoietic cells, and CD45− stromal cells were collected using trypsin and quantified by flow cytometry. Results are the mean values of the ratio of CD19+ to CD45− cells (n = 4). The error bars indicate the SD of the mean. *Mean value is statistically different from that of coculture with unstimulated stromal cells (P<.05).
Primary FL B cells bound to BM-MSCs and pretreatment of stromal cells with TNF/LT significantly enhanced this adhesion (P<.05, n = 4; Figure 6C), as described (see “BM-MSCs could differentiate into FRCs”) for normal tonsil leukocytes (Figure 4C). Collectively, these results suggest that TNF/LT essentially modulates the capacity of BM-MSCs to induce malignant B-cell adhesion but not migration.
Tonsil- and BM-derived stromal cells support the survival of primary FL cells
To further assess the biologic relevance of our data, we investigated whether Resto cells, T-MSCs, and BM-MSCs could protect primary FL cells from spontaneous apoptosis in vitro.
CD19+ primary FL cells purified from 6 patients at diagnosis were cultured with or without stromal cell layers, and the absolute numbers of TOPRO-3−CD45+ viable B cells remaining at the end of the culture were compared (Figure 7). Resto cells, T-MSCs, and BM-MSCs significantly enhanced the mean number of viable FL cells at day 5 by 69%, 86%, and 47%, respectively (P<.05, n = 6). Moreover, pretreatment with TNF/LT strengthened the supportive effect of LN- and BM-derived stromal cells on tumor B-cell survival (P<.05, n = 6). Indeed, stimulation of stromal cells by TNF/LT before coculture enhanced the number of viable FL cells by 2.2-fold for Resto cells, 2.3-fold for T-MSCs, and 2.4-fold for BM-MSCs compared with the culture without stromal cells. Interestingly, neither BM- nor LN-derived stromal cells stimulated purified FL B-cell proliferation in 5 of 5 patients at diagnosis. On the contrary, Resto cells, BM-MSCs, and T-MSCs induced a strong proliferation of tumor B cells obtained from one FL patient in relapse. This proliferation was further enhanced following stromal cell treatment with TNF/LT (Table S1).
Resto cells and MSCs pretreated with TNF/LT support the survival of primary FL B cells. CD19+ B cells purified from 6 patients with FL were cultured in RPMI1640/10% FCS for 5 days, alone or with confluent Resto cells (A), cloned T-MSCs (B), or BM-MSCs (C) pretreated or not with TNF/LT for 7 days. Tumor B-cell viability was determined using CD45 and TOPRO-3 staining. Data are expressed as the absolute number of viable tumor cells (CD45+TOPRO-3−) quantified using calibrated microbeads. *Represents a significant difference in the number of viable FL cells between the compared groups (P<.05).
Resto cells and MSCs pretreated with TNF/LT support the survival of primary FL B cells. CD19+ B cells purified from 6 patients with FL were cultured in RPMI1640/10% FCS for 5 days, alone or with confluent Resto cells (A), cloned T-MSCs (B), or BM-MSCs (C) pretreated or not with TNF/LT for 7 days. Tumor B-cell viability was determined using CD45 and TOPRO-3 staining. Data are expressed as the absolute number of viable tumor cells (CD45+TOPRO-3−) quantified using calibrated microbeads. *Represents a significant difference in the number of viable FL cells between the compared groups (P<.05).
Discussion
With regard to lymphoid organ stromal cells, previous studies have essentially focused on FDCs, and little attention has been given to FRCs. However, accumulating evidence indicates that the paracortex and the GC microenvironments are coordinated elements of a single functional unit that will be reorganized following physical contact with activated lymphocytes. Thomazy et al identified modulation of the FRC meshwork in human FL that showed a more pronounced pattern of transglutaminase staining compared with normal secondary follicle.24 In addition, FDC-like stromal cells found in FL LNs display an undifferentiated phenotype, close to that of FRCs.23 These data suggest that FRCs could be an important, although unexplored, stromal cell subset in FL. In the case of BM involvement, LN-like reticular stromal cells with heterogeneous phenotype are admixed with malignant B cells among nodular aggregates, suggesting that the interactions between FL cells and the medullar stromal cell compartment favor lymphoma development.26 Whereas CD21hiCD35hi functional FDCs cannot be maintained in culture in vitro, a recent study described an elegant model of mouse FRCs.17 FRC-lymphocyte interplay promotes synthesis of extracellular reticular meshwork made up of ER-TR7, fibronectin, and laminin fibers, production of inflammatory chemokines, and migration and adhesion of T cells and iDCs. The primary goal of our study was to establish such a biologically relevant in vitro model in humans. We also explored the role played by fully characterized LN- and BM-derived FRCs in the growth of lymphoma B cells.
First, we generated Resto stromal cells from LNs. Resto cells were defined as a homogeneous population of CD45−CD14− CD90+CD73+CD105+ cells that never expressed at the protein or RNA levels the FDC-specific markers CD21 long isoform, CD35, CD23, or CXCL13 even after stimulation with TNF/LT, CD40 signal, or coculture with tonsil leukocytes (Figure S1 and data not shown). In addition, Resto cells secreted, in response to a sustained stimulation with TNF/LT, a dense reticular meshwork of interconnected fibers made of extracellular matrix components including fibronectin and transglutaminase. Interestingly, iDCs were already strongly adherent to untreated Resto cells. These data correlate well with the demonstration that LN-resident iDCs directly interact with reticular fibers in steady-state conditions.5 Collectively these results argue for an FRC phenotype, very similar to that previously described in the mouse model.17 Our study is consistent with numerous previous studies showing that human and murine LN–derived stromal cells are essentially unable to express FDC markers in vitro.3,17,34,35 The incapacity of Resto cells and BM-MSCs to differentiate into FDCs raises at least 3 hypotheses. First, the differentiation potential of FRCs and MSCs into FDCs is lost during in vitro culture. Second, FDCs and FRCs derived from distinct mesenchymal precursors. Third, whereas TNF/LT combination is sufficient to drive MSC differentiation into FRCs, additional unidentified signals are required to induce the MSC-FDC transition. In agreement with this last postulate, a recent work elegantly demonstrated that signals others than TNF/LT, like those induced in vivo by a stimulation by CD40L or antigen, are required for proper lymphoid organization including induction of FDC-containing B-cell follicles.19
CXCL9, CXCL10, and CCL5 inflammatory chemokines, but also CCL19 lymphoid chemokine, were induced following stimulation of Resto cells by TNF/LT. Mouse FRC lines are unable to produce lymphoid chemokine in vitro, even after treatment with TNF, LT, and anti-LTβR antibody.17 However, lymphoid chemokines are produced by HEVs in mice, whereas in humans they seem to be essentially expressed by FRCs.11,12 Surprisingly, we cannot detect CCL21 in LN and BM stromal cells stimulated with TNF/LT. CCL19 and CCL21 displayed some differing activities in vitro and in vivo.36 Moreover, although the expression of CCL19 is completely abrogated in the nasal-associated lymphoid tissue of LTα−/− mice, CCL21 expression is not impaired, suggesting that CCL21 could be regulated independently of LT.37
TNF and LT collaborate to induce a full FRC phenotype in human stromal cells. This could be linked to the activation of 2 different signaling pathways that coordinate the expression of only partially overlapping sets of genes.38 TNFR1 induces the canonical NF-κB pathway involving the IKK2/NEMO complex, whereas LTβR also activates the alternative NF-κB pathway that mediates an IKK2-independent, IKK1-dependent translocation of p52:RelB heterodimers. Complete loss of LNs is observed in mice deficient in LTβR, whereas TNFR1 deficiency results in a less severe phenotype. Disruption of both TNF and LT signaling in TNF/LTΔ3 mice results in more severe defects of the lymphoid ER-TR7+ meshwork than a single TNF or LT knockout.39 Collectively, these studies demonstrate that TNF and LT are both required for the proper construction of the FRC and FDC networks.
Even if the mesenchymal origin of LN-stromal cells remains controversial,40 studies on LN organogenesis in mice suggest that they derive from a common CD106+CD45− progenitor. We provide here the first direct demonstration that adult human BM-MSCs could give rise to fully competent FRCs in the presence of TNF/LT. Moreover, we were able to isolate a population of resident MSCs from secondary lymphoid organs. T-MSCs shared all the properties of BM-MSCs and could also acquire an FRC phenotype under TNF/LT stimulation. These data support the hypothesis that FRCs may arise from resident mesenchymal precursors in actively stimulated lymphoid tissues. Some evidence indicates that BM-MSCs migrate to LNs in steady-state or inflammatory conditions. Strikingly, donor MSCs were recently detected in the LNs of a patient treated for an acute graft-versus-host disease by intravenous infusion of allogeneic MSCs.41 The relative contribution of resident and recruited MSCs to the pool of FRCs may depend on several individual factors including age but also inflammatory, infectious, or tumor background.
Interaction between BM stromal cells and B cells has never been explored in the context of a GC-derived mature B-cell neoplasia. Specialized microenvironmental niches have been recently identified within normal BM.42 Among them, stage-specific niches, including different stromal cell subsets, provide appropriate signals that ensure either B-cell lymphopoiesis or long-term survival of fully mature plasma cells. Similarly, BM stromal cells support tumor development and protect tumor cells against chemotherapy-induced cell death in B-lineage acute lymphoblastic leukemias and multiple myeloma.43-46 Of interest, increasing attention has focused on the key role played by the BM microenvironment in the natural history of B-cell chronic lymphocytic leukemia (B-CLL), a mature B-cell neoplasia.47-49 BM infiltration is a common feature in FL. We describe for the first time a bidirectional interaction between BM-MSCs and FL B cells. BM-MSCs mediated primary FL cell migration and adhesion. As previously suggested,32 CXCL12 was a pivotal factor in the recruitment of malignant GC-derived B cells because CXCR4 blockade completely abrogated their migration toward BM-MSCs. However, lymphoma cell migration in vivo is a complex process resulting from the integration of multiple signals, and other chemokines present in stromal cell supernatant could probably modulate CXCL12-induced chemotaxis. As an example, CCL2 has been described as a potent FL cell chemoattractant only in combination with CXCL1250 and we were able to detect high levels of CCL2 mRNA in LN- and BM-derived stromal cells (data not shown). Similarly, within LNs, production of CXCL13 by FDCs and CXCL12 by FRCs synergistically directs the accumulation of CXCR4+CXCR5+ FL cells.51
BM-MSCs efficiently protected GC-derived lymphoma cell lines from serum deprivation–induced cell death to the same extent as LN-derived Resto cells. This supportive effect was further enhanced after a 7-day treatment with TNF/LT, which induced a full FRC phenotype. Likewise, these data could be extended to primary FL cells that spontaneously undergo apoptosis in vitro. Of note, Resto cells, BM-MSCs, and T-MSCs did not promote proliferation of FL B cells at diagnosis. FL is the most frequent indolent lymphoma and is associated primarily with abnormal survival rather than extensive proliferation, in particular due to the overexpression of the antiapoptotic protein bcl-2 through the t(14;18) translocation.47,52 Interestingly, the only patient showing malignant B-cell proliferation in contact with stromal cells was one with FL in relapse. To evaluate if this phenomenon is really linked to disease progression, it would be meaningful to extend this study to other patients with relapsed FL and to patients with diffuse large B-cell lymphoma (DLBCL), an aggressive lymphoma that could arise as a transformation of indolent FL.
Using nonexhaustive quantitative PCR experiments, we identified B-cell activating factor of the TNF family (BAFF), hepatocyte growth factor (HGF), and IL-15 as highly expressed by Resto cells and BM-MSCs (not shown). Among them, IL-15 mRNA was the only one that was clearly up-regulated by treatment with TNF/LT. These 3 cytokines are produced by FDCs and are involved in normal B-cell proliferation and survival.53-57 In addition, at least BAFF and HGF contribute to NHL pathogenesis.58-61 The detailed role of these soluble factors in the stromal cell–dependent inhibition of FL B-cell death remains to be elucidated, as well as the place of direct cell contact. Similarly, CXCL12 has been involved in cell survival and proliferation in B-CLL and EBV-associated lymphoproliferations.62,63 Further investigation would be useful to evaluate its potential role in FL pathogenesis.
Conversely, tumor B cells, unlike normal B cells, could induce an FRC phenotype on BM-MSCs. As previously described by others,64 we found, using quantitative RT-PCR, that purified FL B cells express, unlike stromal cells, high levels of transcripts for TNF, LTα, and LTβ (data not shown). These cytokines could thus collaborate in vivo for the induction of FRC differentiation.
Collectively, our results provide new insights into the role of the stromal microenvironment in FL pathogenesis, both in the lymphoid and the medullar compartments. In addition, the in vitro culture system described here provides a promising biologically relevant tool to further investigate the mechanism of action of antitumor reagents and to develop new drugs targeting the interactions between lymphoma cells and their environment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: P.A.-T. designed and performed research, analyzed data, and wrote the paper; H.M.-E.H. performed research and analyzed data; R.J. and C.M. performed research; D.M. performed research and analyzed data; S.C.-M. performed research; T.G. reviewed the manuscript; T.L. contributed patients and helped to raise funds; T.F. reviewed the manuscript and helped to raise funds; K.T. was senior author, designed and supervised research, analyzed data, and wrote the paper.
P.A.-T. and H.M.-E.H. contributed equally to this work.
We thank Dr Frederic Deschaseaux and Dr Cornelis De Groot for technical assistance and helpful scientific discussions. The authors are grateful to Dr Christophe Ruaux for providing tonsil samples, Pr Alain Leguerrier and Dr Arnaud Thauran for help with human bone marrow aspirates, and Dr Céline Pangault and Dr Patrick Tas for very efficient sample management. Thanks also to the Institut Fédératif de Recherche (IFR) 140 microscopy platform (Rennes, France).
This work was supported by grants from the Région Bretagne (Accueil et émergence de nouvelle équipe en Bretagne), the Fondation pour la Recherche Médicale (FRM), the Ligue Régionale Contre le Cancer, the Association pour le Développement de l'Hémato-Oncologie (ADHO), the Université Rennes 1, and the Cancéropôle Grand Ouest.