To the editor:
The mechanisms involved in the clearance from plasma of approximately 1012 copies of hepatitis C virus (HCV) produced daily1 are unclear. The use of the anti-CD20 monoclonal antibody rituximab, which reversibly depletes B cells, in HCV-related mixed cryoglobulinemia2 afforded an opportunity to study the potential role of humoral immunity.
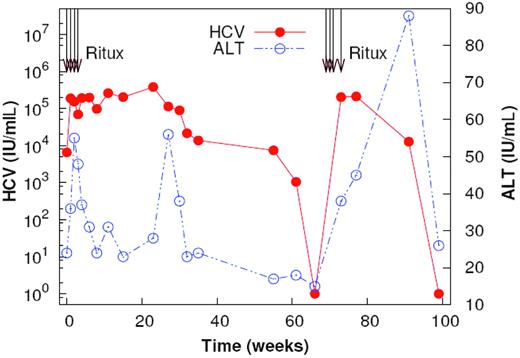
We evaluated chronologic changes in plasma HCV RNA, and alanine aminotransferase (ALT) levels, in a 40-year-old male with chronic genotype 1 HCV-related cryoglobulinemia after 2 courses of rituximab (375 mg/m2 per week for 4 weeks, 70 weeks apart). Antiviral therapy, including pegylated interferon, reduced baseline viral load (VL) from 122 000 IU/mL to less than 7000 IU/mL. Therapy was maintained throughout. VL increased from less than 7000 IU/mL to 252 893 IU/mL within 2 weeks of starting rituximab infusions. It continued to rise progressively to 379 000 IU/mL by week 23 (Figure 1). B-cell markers, including CD20, CD19, CD21, IgM, IgD, IgG, and CD72,3 were absent from peripheral blood up to week 13. Thereafter, B-cell frequency increased to 8% by week 32. Although there is evidence for HCV replication in B cells,4 the continued rise in VL after B-cell depletion argues against this as a source of HCV RNA increase.
Plasma HCV RNA and ALT changes after rituximab infusions. Two courses of rituximab were given at week 0 and week 70. Plasma HCV RNA and ALT are shown. HCV RNA levels increase rapidly after the first dose of rituximab. Transient increases in ALT are observed at the initiation of treatment and with the reappearance of B cells.
Plasma HCV RNA and ALT changes after rituximab infusions. Two courses of rituximab were given at week 0 and week 70. Plasma HCV RNA and ALT are shown. HCV RNA levels increase rapidly after the first dose of rituximab. Transient increases in ALT are observed at the initiation of treatment and with the reappearance of B cells.
Following reappearance of B cells, VL decreased starting from week 23 to less than 615 IU/mL by week 63. A second 4-week course of rituximab infusions at week 70 similarly resulted in a prompt, more than 2-log rise in VL to 202 000 IU/mL (Figure 1).
On both occasions, rituximab increased ALT levels transiently. This suggests increased de novo infection5 and that B cells may play a protective role, thus accounting for the more rapid disease progression in immunodeficiency disorders.6 A second ALT level flare at week 27 coincided with the reappearance of B cells and HCV RNA decline. We speculate that this might indicate antibody-dependent cellular cytotoxicity.
Titers of anti-E1/E2 HCV neutralizing antibodies (nAbs) were measured in plasma using pseudotype viruses expressing heterologous HCV envelope glycoproteins.7 Levels were unaffected by B-cell depletion. As others have noted,8 plasma IgM fell by more than 50% and continues to be depressed; IgG and IgA remain stable. Stimulated B cells account for most of the IgM circulating in plasma. Furthermore, circulating HCV in chronic infection is predominantly bound to IgM.9 In this patient on interferon treatment, the rapid rituximab-induced increase in VL could reflect loss of nonneutralizing IgM antibodies secreted by stimulated naive or memory B cells. Possible mechanisms for HCV clearance might include virus opsonization and complement activation.10
HVR1 sequences were cloned from polymerase chain reaction (PCR) products at various times between weeks 0 and 32. The serine in position 408, present at low frequencies for the first 4 weeks, increased to 55% at week 4 and to 100% at week 6, decreasing slightly thereafter. Reduced HVR1 sequence diversity with depletion of B cells suggests that humoral immunity can exert immune selective pressure on HCV envelope.
These preliminary data are consistent with a significant effect of B cells on the control of plasma hepatitis C viremia.
Conflict-of-interest disclosure: The authors declare no competing financial interests.