Abstract
Rapid, profound, and selective depletion of memory CD4+ T cells has now been confirmed to occur in simian immunodeficiency virus (SIV)–infected adult macaques and human immunodeficiency virus (HIV)–infected humans. Within days of infection, marked depletion of memory CD4+ T cells occurs primarily in mucosal tissues, the major reservoir for memory CD4+ T cells in adults. However, HIV infection in neonates often results in higher viral loads and rapid disease progression, despite the paucity of memory CD4+ T cells in the peripheral blood. Here, we examined the immunophenotype of CD4+ T cells in normal and SIV-infected neonatal macaques to determine the distribution of naive and memory T-cell subsets in tissues. We demonstrate that, similar to adults, neonates have abundant memory CD4+ T cells in the intestinal tract and spleen and that these are selectively infected and depleted in primary SIV infection. Within 12 days of SIV infection, activated (CD69+), central memory (CD95+CD28+) CD4+ T cells are marked and persistently depleted in the intestine and other tissues of neonates compared with controls. The results in dicate that “activated” central memory CD4+ T cells are the major target for early SIV infection and CD4+ T cell depletion in neonatal macaques.
Introduction
Studies have confirmed that both simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV) rapidly and selectively infect and eliminate activated memory CD4+ T cells in rhesus macaques and humans, respectively. The vast majority of these memory CD4+ T cells are infected and eliminated from all lymphoid tissues in adult primate hosts.1-5 Because the intestine contains the majority of the activated memory CD4+ T cells in the body, the depletion of CD4+ T cells in this site is much more profound than in peripheral lymphoid tissues.6 Moreover, recent studies in adult macaques have strongly implicated direct infection of these cells (rather than bystander apoptosis) as the mechanism for this loss, because up to 60% of the memory CD4+ T cells in the intestine were shown to be infected just prior to the depletion in acute SIV infection.4,5
It has been proposed that this rapid and dramatic loss of mucosal CD4+ T cells is due to the high proportion of activated memory CD4+ T cells expressing CCR5 in intestinal CD4+ T cells.1,6 In addition, studies suggest that mucosal T cells have much higher levels of activation, which is required for optimal viral replication and lytic infection.1,6
Interestingly, HIV-infected children often have a more rapid disease course and higher viral loads than adults.7-10 This is counterintuitive, because children (particularly infants) would be expected to have fewer antigenic exposures and thus fewer activated memory CD4+ T cells than adults. In fact, data in a variety of species show that peripheral blood and lymph node lymphocytes of neonates are primarily naive. This would suggest a paucity of viral target cells required for optimal HIV/SIV replication. However, most existing data on T-cell subsets in neonates are based on examination of peripheral blood and not mucosal sites, which represent the primary target for acute SIV and HIV infection in adults. Therefore, we have been examining mucosal T-cell subsets in normal and SIV-infected neonatal macaques.
We have previously shown that infant macaques, like infant children, have much higher percentages of CD4+ T cells at birth.11-13 Surprisingly, neonates also have substantial percentages of CD4+ T cells with an activated (HLA-DR+) phenotype in the intestinal lamina propria as compared with peripheral lymphoid tissues, even when examined on the day of birth.13 Moreover, profound and selective depletion of intestinal lamina propria CD4+ T cells occurs in SIVmac239-infected neonatal macaques within 21 days of infection, which is preceded by widespread SIV infection of cells in this compartment.13 However, prior studies did not determine whether the loss of CD4+ T cells was selective for central or effector memory CD4+ T-cell subsets, nor was a thorough assessment of other activation markers (CD25, CD69) performed to specifically determine which cell subsets were being eliminated. We also did not phenotype SIV-infected cells in previous studies and thus could not demonstrate that the loss of CD4+ T cells in the intestine was mediated through direct viral infection of these cells. Because new strategies and markers for defining naive and memory-cell subsets in rhesus macaques have recently emerged, as well as improvements in technologies for defining cell subsets directly infected with SIV, the current studies were performed to more carefully delineate the phenotype and activation status of the CD4+ T cells that are being eliminated in early SIV infection.
The data presented here demonstrate that, as in adults, CD4+ T cells in the intestinal lamina of neonatal macaques are unique compared with peripheral lymphoid tissues, in that they consist mostly of activated memory cells as defined by coexpression of the memory markers CD45RO and CD95. Moreover, we demonstrate that early SIV infection selectively infects activated memory CD4+ T cells in neonates and that, similar to infection of adult macaques, these cells are rapidly and selectively eliminated. Finally, we demonstrate that CD45RO+ T cells are preferentially infected in the intestine, confirming that the selective loss of memory CD4+ T cells in the intestine and other tissues is primarily the result of direct viral infection rather than bystander mechanisms.
Materials and methods
Animals and virus
Tissues from 16 infected and 15 uninfected neonatal rhesus macaques (Macaca mulatta) were obtained from the New England and Tulane National Primate Research Centers. Macaques were obtained from their dams at birth and hand reared on formula thereafter. Experimental animals were intravenously infected with 100 TCID50 (Tulane) or 50 ng p27 (New England) of SIVmac251 within 24 hours of birth, and humanely killed at 3, 7, 12, 14, 21, or 50 days after infection for comparison with age-matched uninfected controls as shown in Table 1 Uninfected controls were killed for tissue collection at 3, 7, 12, 14, 21, or 50 days of age (Table 1).
All monkeys were maintained in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care, and all studies were approved by the Tulane and Harvard Institutional Animal Care and Use Committees.
Tissue collection and analysis
For routine histopathology, immunohistochemistry, and in situ hybridization, complete sets of tissues (multiple sections of intestine, multiple lymph nodes, spleen, etc) were harvested and preserved in 10% neutral-buffered formalin. Portions of spleen; axillary and mesenteric lymph nodes; and 6- to 8-cm long sections of the jejunum, ileum, and colon were also collected fresh in complete RPMI 1640 medium and immediately transported to the laboratory for lymphocyte isolation.
Lymphocytes from the jejunum, ileum, colon, lymph nodes (axillary and mesenteric), and spleen were isolated as previously described.13,14 Purified cell suspensions were adjusted to 107 cells/mL and 100-μL aliquots (106 cells) were stained using appropriately diluted concentrations of antibody for 30 minutes at 4°C. Samples from all macaques were examined by 4-color flow cytometry, but 10 were also examined by 8-color flow cytometry as described in Table 2 Details of all animals examined in this study and the staining panels used to examine them are shown in Tables 1 and 2.
Whole blood and spleen samples were stained using a whole blood lysis protocol as previously described.15 Cell preparations of lymph nodes, intestine, and other tissues were adjusted to 107 cells/mL, and 100-mL aliquots (106 cells) were stained for 30 minutes at 4°C with appropriately diluted concentrations of antibodies. Cells were then washed in PBS and fixed overnight in 2% paraformaldehyde. Samples were acquired on a fluorescence-activated cell sorting (FACS) Calibur for 4-color samples or a FACS Aria flow cytometer (Becton Dickinson, San Jose, CA). Data were analyzed with Flowjo software (Tree Star, Ashland, OR). At least 10 000 lymphocytes were collected for analysis from each sample, and data were analyzed by gating through lymphocytes and then cells of interest as described.
Quantifying memory CD4+ T cells in intestinal tissues
Immunohistochemistry for memory CD4+ cells was examined and quantified on intestinal tissues from 5 infected macaques (day 14, and 2 each at days 12 and 21 after infection) and 5 uninfected (7, 12, 14, and 2 at 21 days old) neonates using the monoclonal antibody OPD4 (Dako, Carpinteria, CA), which has been reported to react specifically with CD45RO expressed on CD4+ memory T cells.16,17 Briefly, paraffin sections were deparaffinized, and antigens were unmasked using high-temperature antigen retrieval consisting of heating slides in a steam bath chamber (Flavor Scenter Steamer Plus; Black and Decker, Hunt Valley, MD) with 0.01 M citrate buffer pH 6.0 for 20 minutes, cooled, and washed twice in phosphate-buffered saline (PBS). Slides were blocked with peroxidase blocking reagent (Dako, Glostrup, Denmark) for 10 minutes, washed in PBS, further blocked with serum-free protein block (Dako) for 30 minutes, and incubated with the primary antibody for 1 hour at room temperature. Slides were then washed with PBS and developed using a Vectastain ABC peroxidase kit (Vector Laboratories, Burlingame, CA) and 3,3-diaminobenzidine DAB (Biocare Medical, Concord, CA). CD45RO+ cells were automatically counted in sections of defined areas using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). From each macaque, the number of positive cells in the lamina propria of at least 8 × 200 sections were counted and expressed as the number of positive cells per square millimeter of lamina propria.
Phenotyping SIV-infected cells in tissues by in situ hybridization and immunohistochemistry
To identify cells that harbored viral mRNA, nonradioactive in situ hybridization was combined with immunohistochemistry on formalin-fixed, paraffin-embedded sections of jejunum. Briefly, 5-mm sections were cut and adhered to sialinized glass slides. After deparaffinization in xylene, rehydration in PBS, and antigen retrieval with steam (citrate buffer as described in “Quantifying memory CD4+ T cells in intestinal tissues”) the sections were acetylated and hybridized with digoxigenin-labeled antisense SIV riboprobes (Lofstrand Labs, Gaithersburg, MD) encompassing essentially the entire SIV genome as previously described.18 Labeled cells were visualized using horseradish alkaline phosphatase-conjugated sheep antidigoxigenin antibodies. SIVRNA+ cells were detected by the 2-hydroxy-3-napthoic acid-2-phenylanalide phosphate (HNPP) Fluorescent Detection Set (Roche Diagnostics, Palo Alto, CA). For dual labeling, SIV-infected cells detected by this technique also stained for CD45RO expression by incubating slides with the OPD4 monoclonal antibody, followed by incubation with Alexa Fluor 488 (green) labeled secondary antibody (goat anti–mouse IgG1). After staining, slides were washed, mounted with fluorescent mounting medium, and visualized using a confocal microscope.
Confocal microscopy
Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with 3 lasers (Leica Microsystems, Exton, PA). Individual optical slices represent 0.2 mm, and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. NIH Image (version 1.62) and Adobe Photoshop (version 7.0; San Diego, CA) were used to assign colors to the channels collected: HNPP/Fast Red is a substrate that fluoresces red when exposed to a 568-nm wavelength laser and Alexa 488 (Invitrogen, Carlsbad, CA) fluoresces green.
Statistics
Statistical analyses were performed with a paired Student t test using GraphPad Prism software (GraphPad Software, San Diego, CA). Absolute numbers of CD45RO+ cells in tissues were compared between all infected and noninfected animals. P value less than .05 were considered significant.
Results
Neonatal lymphocytes from the intestine have much higher CD95+CD28+ coexpression than those from lymph nodes and blood
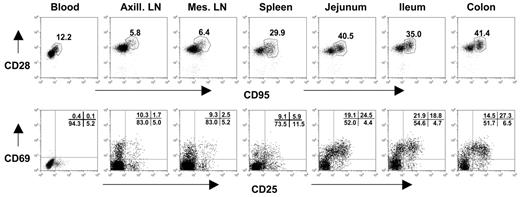
The expression of CD28 and CD95 has been used to define distinct subsets of CD4+ and CD8+ T lymphocytes in rhesus macaques.19 Naive T cells can be identified by their expression of CD28 and lack of CD95 expression. In contrast, central memory cells have been defined as CD95+CD28+, and effector memory cells have been described as primarily CD95+ and CD28−.19 As in human infants,20 we found that peripheral blood CD4+ T cells of neonatal macaques were mostly naive as determined by the absence of CD95 expression. Only a mean of 10.2% (range, 5.0%-16.4%) of peripheral blood CD4+ T cells of uninfected neonates coexpressed CD95 (Table 3) In contrast, the immunophenotype of CD4+ T cells isolated from the intestines of neonates, particularly in the lamina propria of the jejunum, was significantly different (Figure 1). Between 40.7% and 96.1% of intestinal CD4+ T cells expressed CD95, indicating that neonatal intestinal lymphocytes have significantly more central memory cells compared with peripheral lymphoid tissues (blood, lymph nodes, and spleen) (Table 3; Figure 1).
Flow cytometry dot plots demonstrate coexpression of CD95 and CD28 (top) and CD25 and CD69 expression (bottom) on CD4+ T cells from a representative neonate (GB14, 7 days old). Like adults (data not shown), neonates have large numbers of memory (CD95+) and activated (CD25+CD69+) CD4+ T lymphocytes in the intestinal tract (jejunum, ileum, colon) compared with peripheral lymphoid tissues (spleen, lymph nodes, and blood). Central memory cells are defined by CD95 and CD28 coexpression (top, polygonal gates). Note that there are no effector memory cells (CD95+, CD28−) evident in any tissues. Plots were generated by gating on CD4+ lymphocytes.
Flow cytometry dot plots demonstrate coexpression of CD95 and CD28 (top) and CD25 and CD69 expression (bottom) on CD4+ T cells from a representative neonate (GB14, 7 days old). Like adults (data not shown), neonates have large numbers of memory (CD95+) and activated (CD25+CD69+) CD4+ T lymphocytes in the intestinal tract (jejunum, ileum, colon) compared with peripheral lymphoid tissues (spleen, lymph nodes, and blood). Central memory cells are defined by CD95 and CD28 coexpression (top, polygonal gates). Note that there are no effector memory cells (CD95+, CD28−) evident in any tissues. Plots were generated by gating on CD4+ lymphocytes.
Memory CD4+ T cells (CD95+) were further subdivided based on the coexpression of CD28 (central memory cells) or the lack thereof (effector memory cells). Essentially, all CD4+ T cells in tissues of neonates were either naive (CD95−) or central memory cells (CD95+CD28+), and very few effector memory CD4+ T cells (CD95+CD28−) were detected in any of the tissues from any neonates examined in this study (Figure 1; Table 3). Only the spleen had significant levels of CD4+CD95+CD28− cells, and these were still very rare, because only one neonate (GA98) had greater than 5% of effector memory CD4+ T cells (gated through total CD4+ T cells) in the spleen (Table 3).
Majority of “activated” CD4+ T cells are in the intestinal tract of neonates
Because both HIV and SIV optimally replicate in activated memory CD4+ T cells, we used 4- to 8-color flow cytometry to examine the expression of the early activation marker CD69 and a late activation marker CD25 (the IL-2 receptor) to distinguish activated from resting T-cell subsets in different tissues of neonates. CD69 is an early and reliable marker of lymphocyte activation.21 CD25 is considered to be expressed on activated, but not resting T cells as well as a subset of T-regulatory cells that may be involved in suppressing immune responses.22,23 Because CD69 is considered a definitive marker of activation, and because most of the CD25+ cells observed in neonates were CD25low or CD25intermediate (Figure 2) rather than CD25high (one criteria for defining Treg cells), we are defining CD69+ T cells, CD25+ T cells, or both here simply as activated cells in this study.
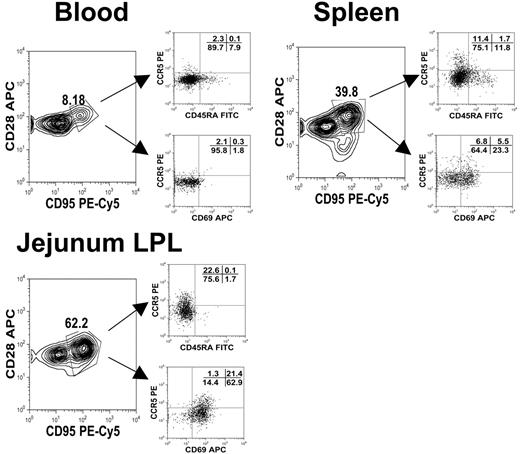
Polychromatic (8 color) flow cytometry demonstrating that central memory (CD95+CD28+) CD4+ T cells from different tissues have different expression patterns of activation markers (CD25, CD69) as well as CCR5 and CD45RA. Plots were gated through lymphocytes and then through CD3+CD4+ T cells from tissues from an uninfected neonate (GA98, 14 days). Note that central memory (CD95+CD28+) CD4+ T cells from the intestine have much higher CD69 and CCR5 expression than those from the spleen or blood.
Polychromatic (8 color) flow cytometry demonstrating that central memory (CD95+CD28+) CD4+ T cells from different tissues have different expression patterns of activation markers (CD25, CD69) as well as CCR5 and CD45RA. Plots were gated through lymphocytes and then through CD3+CD4+ T cells from tissues from an uninfected neonate (GA98, 14 days). Note that central memory (CD95+CD28+) CD4+ T cells from the intestine have much higher CD69 and CCR5 expression than those from the spleen or blood.
As in adult macaques,1,15 a much higher percentage of CD4+ T cells in the intestine of neonatal macaques coexpressed both CD69 and CD25 compared with any other lymphoid tissue examined. Between 8% and 44% of jejunum CD4+ T cells were CD25+CD69+ compared with less than 1% of blood, 2.3% of lymph node, and 3% of spleen CD4+ T cells (Table 4) Individually, between 12.2% to 49.3% and 21.9% to 95.8% of the CD4+ T cells isolated from the intestine expressed either CD25 or CD69, respectively (Table 4), indicating that neonates are born with a relatively high expression of these activation molecules on their CD4+ T cells in the intestine. In addition, the majority of CD69+ cells, at least in the intestine, coexpressed CD25, suggesting that these were indeed activated cells rather than T-regulatory cells. In contrast, only 3.4% to 8.7% of peripheral blood, 2.2% to 9.9% of lymph node, and 3.7% to 17.4% of spleen CD4+ T cells coexpressed CD25, and, interestingly, in most of these peripheral lymphoid tissues, CD25+ cells did not coexpress CD69. In fact, very little CD69 expression was detected on peripheral blood CD4+ T cells in general (Table 4).
Phenotype of central memory (CD28+CD95+) CD4+ T cells in neonates
As previously mentioned, CD4+ T-lymphocyte subsets may be defined as central memory (CD28+CD95+) and effector memory (CD28−CD95+) CD4+ T cells.19 However, as shown in Table 3, few effector CD4+ T cells were detected in these infants, regardless of the tissue examined. Essentially all memory CD4+ T cells in tissues of normal (uninfected) neonates coexpressed both CD95 and CD28, suggesting they were “central memory” cells. Using polychromatic (8-color) flow cytometry, we further characterized these cells in tissues. In the blood, rare CD3+CD4+ T cells with a central memory phenotype (CD95+CD28+) coexpressed CD69, but about 29% of the central memory CD4+ T cells in the spleen coexpressed CD69. In contrast, around 85% of the central memory (CD95+CD28+) CD4+ T cells from intestinal tissues coexpressed CD69 (Figure 2). Furthermore, all central memory CD4+ T cells in the jejunum of neonates lacked CD45RA, and many coexpressed CCR5 (consistent with a previously activated memory phenotype) (Figure 2). Combined, these results suggested that intestinal central memory CD4+ T cells were indeed activated, yet they maintained a central rather than an effector phenotype.
Rapid and selective depletion of activated memory CD4+ T cells occurs in the intestine of SIV-infected neonatal macaques
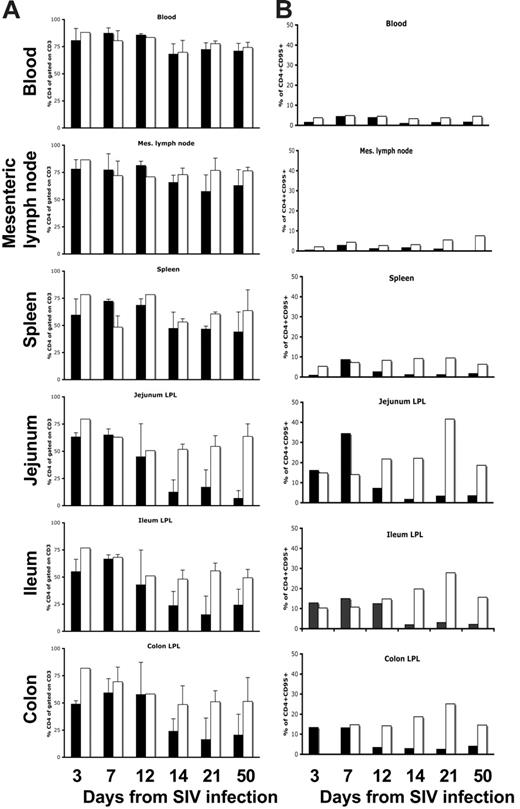
As shown in previous studies uninfected normal neonates have far more CD4+ T cells in their peripheral blood and tissues than do adults.12,13 On infection of neonates with SIV, there was profound and selective depletion of intestinal lamina propria CD4+ T cells in neonatal macaques within 14 to 21 days of infection (Figure 3). As expected from prior studies, CD4+ T cells were markedly depleted in the jejunum, ileum, and colon of neonates within 14 days of infection, yet CD4 cells in the blood and lymph nodes were essentially unchanged (Figure 3). Moreover, analysis of the specific subsets of CD4+ T cells that were depleted indicated this loss was selective for memory CD4+ T cells. Figure 4 shows that a marked and almost complete loss of CD4+CD95+ memory cells occurred in the intestine within 12 days of infection compared with age-matched controls (Figure 4A). Moreover, this loss was highly selective for central memory (CD95+CD28+) CD4+ T cells (Figure 4B). Although the majority of CD4 cells in the jejunum of a 21-day-old neonate coexpressed CD95 and CD28, note that most of the CD4+ T cells that remained in the intestine of the age-matched SIV-infected neonate lacked CD95 expression (Figure 4B). Note that this selective loss of central memory CD4+ T cells could also be detected in the spleen and lymph nodes, but the paucity of this subset in these tissues results in lower levels of overall CD4+ T-cell loss in peripheral lymphoid tissues (Figure 4B).
Bar charts demonstrating percentages of total CD4+ T cells and memory (CD95+) CD4+ T cells in various tissues of SIV-infected and uninfected age-matched normal neonates at various stages of acute infection or age. Note that when examining the changes in total CD4+ T cells (A) there is marked depletion of CD4+ T cells in the intestine (jejunum, ileum, and colon) by 14 days of SIV infection, but minimal changes in CD4+ T cells occur in the blood, lymph nodes, or spleen (A). The selective loss of intestinal CD4+ T cells is a direct reflection of the percentage of memory cells in these tissues before infection (B). Note that intestinal tissues have much higher percentages of memory CD4+ T cells before infection and that these are selectively eliminated in acute SIV infection (B). Data represent percentages of CD4+ cells gated through CD3+ T cells (A) or CD4+CD95+ T cells gated through lymphocytes (B) from groups (standard error bars) or individual animals as described in Table 1. SIV-infected (▪) and uninfected (□) age-matched normal neonates.
Bar charts demonstrating percentages of total CD4+ T cells and memory (CD95+) CD4+ T cells in various tissues of SIV-infected and uninfected age-matched normal neonates at various stages of acute infection or age. Note that when examining the changes in total CD4+ T cells (A) there is marked depletion of CD4+ T cells in the intestine (jejunum, ileum, and colon) by 14 days of SIV infection, but minimal changes in CD4+ T cells occur in the blood, lymph nodes, or spleen (A). The selective loss of intestinal CD4+ T cells is a direct reflection of the percentage of memory cells in these tissues before infection (B). Note that intestinal tissues have much higher percentages of memory CD4+ T cells before infection and that these are selectively eliminated in acute SIV infection (B). Data represent percentages of CD4+ cells gated through CD3+ T cells (A) or CD4+CD95+ T cells gated through lymphocytes (B) from groups (standard error bars) or individual animals as described in Table 1. SIV-infected (▪) and uninfected (□) age-matched normal neonates.
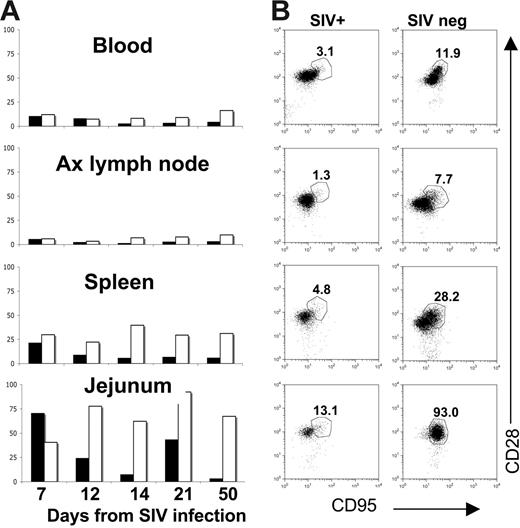
Changes in central memory CD4+ T cells in neonatal tissues on SIV infection. Bar charts (A) indicate percentages of central memory (CD95+CD28+) CD4+ T cells in SIV-infected (solid bars) to age-matched control neonatal macaques (open bars) over time. Note that a selective loss of central memory CD4+ T cells occurs in the jejunum of SIV-infected neonates by 12 days of infection compared with age-matched controls. Bars represent the percentage of CD95+CD28+ cells after gating through CD4+ T lymphocytes as demonstrated in panel B. (B) Dot plots of CD4+ T cells from tissues of a representative SIV-infected neonate (DP53, left column) to an age-matched uninfected neonate (DP44, right column), demonstrating selective loss of CD95+CD28+ CD4+ T cells in all tissues. Plots were generated by gating through lymphocytes and then CD4+ T cells.
Changes in central memory CD4+ T cells in neonatal tissues on SIV infection. Bar charts (A) indicate percentages of central memory (CD95+CD28+) CD4+ T cells in SIV-infected (solid bars) to age-matched control neonatal macaques (open bars) over time. Note that a selective loss of central memory CD4+ T cells occurs in the jejunum of SIV-infected neonates by 12 days of infection compared with age-matched controls. Bars represent the percentage of CD95+CD28+ cells after gating through CD4+ T lymphocytes as demonstrated in panel B. (B) Dot plots of CD4+ T cells from tissues of a representative SIV-infected neonate (DP53, left column) to an age-matched uninfected neonate (DP44, right column), demonstrating selective loss of CD95+CD28+ CD4+ T cells in all tissues. Plots were generated by gating through lymphocytes and then CD4+ T cells.
In addition, there was a marked and selective depletion of highly activated (CD25+CD69+) CD4+ T cells in the intestine of SIV-infected neonates as compared with age-matched controls (Figure 5). Note that, although there is an apparent increase in levels of activated CD4+ T cells as neonates age, there is a marked and selective decrease in this subset in response to SIV infection. There was a mild decline in overall CD4+ T cells in the spleen and lymph nodes, but, similar to the intestine, this decline was selective for those CD4+ T cells having an activated memory phenotype (data not shown). In contrast, the percentages (Figure 3) and absolute numbers (data not shown) of CD4+ cells in the blood of SIV-infected neonates remained similar to those of age-matched controls, consistent with the lack of activated memory CD4+ T cells in this tissue.
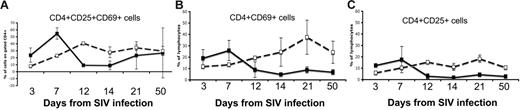
Comparison of CD4+ T cells from the jejunum of neonates. Expression of both CD25 and CD69 (A), CD69 alone (B), or CD25 alone (C) in SIV-infected neonates (solid lines) was compared with age-matched controls (dotted lines). Note that there is a marked and selective loss of activated (CD25+ and/or CD69+) cells in the intestine of all SIV-infected neonates by 12 days of infection.
Comparison of CD4+ T cells from the jejunum of neonates. Expression of both CD25 and CD69 (A), CD69 alone (B), or CD25 alone (C) in SIV-infected neonates (solid lines) was compared with age-matched controls (dotted lines). Note that there is a marked and selective loss of activated (CD25+ and/or CD69+) cells in the intestine of all SIV-infected neonates by 12 days of infection.
Visualization of SIV-infected memory CD4+ T cells in the intestine of SIV-infected neonatal macaque
To visualize changes in memory CD4 T cells in the intestine during SIV infection in neonates, we performed immunohistochemistry for CD45RO expression using the monoclonal antibody clone OPD4, which is reportedly a specific marker for memory CD4+ T cells.16,17 As shown in Figure 6, we observed significantly fewer CD45RO+ cells in the intestine of SIV-infected neonates compared with age-matched normal animals (P < .02). In fact, this difference was even evident in neonates infected for 7 and 12 days (Figure 6C). Combining in situ hybridization for SIV and immunohistochemistry staining for CD45RO, we found that greater than 85% of the SIV-infected cells in the lamina propria of the jejunum had a lymphocyte morphology and expressed CD45RO+ (Figure 6D), verifying that the selective loss of memory CD4+ T cells observed by flow cytometry was due to direct viral infection of these cells. In most SIV+ cells, the intensity of CD45RO staining was very clear, yet some SIV+ cells had weaker CD45RO staining, suggesting that SIV infection could down-regulate the expression of CD45RO (data not shown). If so, this could explain why approximately 15% of the infected cells in tissues lacked CD45RO staining. Alternatively, these infected cells could represent infection of different cell subsets. Regardless, these results clearly demonstrate that SIV infection in neonates selectively targets, infects, and destroys central memory CD4+ T cells, which are abundant in intestinal tissues but rare in peripheral lymphoid tissues.
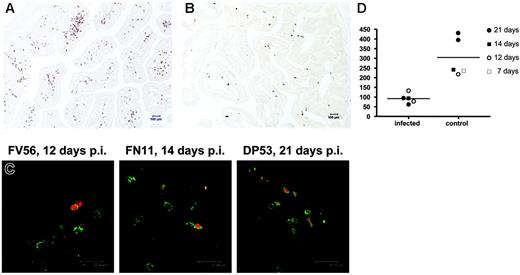
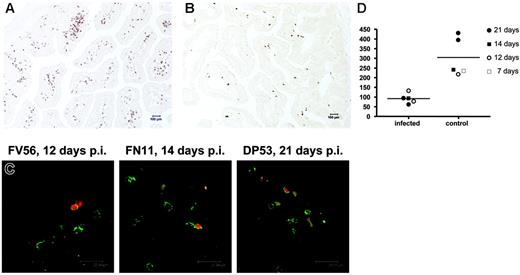
Immunohistochemistry using monoclonal antibody OPD4 demonstrating memory (CD45RO+) CD4+ T cells in the jejunum of an uninfected neonate (21 days old, DP53) and age-matched SIV-infected neonate (21 days after infection, EC73). Note that numerous memory CD4+ T cells are evident in the uninfected jejunum (A) as compared with the SIV-infected neonate (B). (C) Fluorescent in situ hybridization for SIV combined with immunofluorescence for OPD4 (CD4+ memory cells) in the intestine of 3 SIV-infected neonates examined 12 to 21 days after SIV infection. Essentially all of the SIV-infected cells (red) are CD4+CD45RO+ memory cells as defined by reactivity with monoclonal antibody OPD4 (green) using confocal microscopy. (D) Comparison of absolute numbers of CD4+CD45RO+ cells per square millimeter of jejunum lamina propria between SIV-infected and uninfected (control) neonates. Memory CD4+ T cells are markedly decreased in the intestine of all neonates as early as 12 days after SIV infection. Panels A and B were photographed using a Leica DM LB microscope (Leica Microsystems, Bannockburn, IL) with an N-planar 20×/0.40 NA objective equipped with a Spot 3.2.0 digital camera (Diagnostic Instruments, Sterling Heights, MI). Panel C was taken with a Leica TCS SP2 confocal microscope (Leica Microsystems, Exton, PA). NIH image version 1.62 (NIH, Gaithersburg, MD) and Adobe Photoshop (Adobe Systems, San Jose, CA) were used to assign colors to the images.
Immunohistochemistry using monoclonal antibody OPD4 demonstrating memory (CD45RO+) CD4+ T cells in the jejunum of an uninfected neonate (21 days old, DP53) and age-matched SIV-infected neonate (21 days after infection, EC73). Note that numerous memory CD4+ T cells are evident in the uninfected jejunum (A) as compared with the SIV-infected neonate (B). (C) Fluorescent in situ hybridization for SIV combined with immunofluorescence for OPD4 (CD4+ memory cells) in the intestine of 3 SIV-infected neonates examined 12 to 21 days after SIV infection. Essentially all of the SIV-infected cells (red) are CD4+CD45RO+ memory cells as defined by reactivity with monoclonal antibody OPD4 (green) using confocal microscopy. (D) Comparison of absolute numbers of CD4+CD45RO+ cells per square millimeter of jejunum lamina propria between SIV-infected and uninfected (control) neonates. Memory CD4+ T cells are markedly decreased in the intestine of all neonates as early as 12 days after SIV infection. Panels A and B were photographed using a Leica DM LB microscope (Leica Microsystems, Bannockburn, IL) with an N-planar 20×/0.40 NA objective equipped with a Spot 3.2.0 digital camera (Diagnostic Instruments, Sterling Heights, MI). Panel C was taken with a Leica TCS SP2 confocal microscope (Leica Microsystems, Exton, PA). NIH image version 1.62 (NIH, Gaithersburg, MD) and Adobe Photoshop (Adobe Systems, San Jose, CA) were used to assign colors to the images.
Discussion
Selective and rapid depletion of memory CD4+ T cells has now been described in SIV-infected adult macaques and HIV-infected adult humans by multiple groups.2,4,5,15,24,25 In this study, we examined the immunopathogenesis of SIVmac251 infection in various lymphoid tissues of neonatal macaques to determine whether these cells were also the major targets for primary SIV infection of infant macaques. This study demonstrates that SIVmac251 infection selectively infects and depletes CD4+ T cells having an activated (CD25+CD69+) and central memory (CD95+CD28+) phenotype in tissues of neonates, despite the relative “absence” of these cells in the peripheral blood. Because this phenotype is abundant in the intestine but rare in peripheral lymphoid tissues, this results in a massive depletion of CD4+ T cells in the former. We thus demonstrate that, as in adults, the intestinal tract of neonates contains the majority of the memory CD4+ T cells in the body and that these are selectively infected and depleted in primary SIV infection. Moreover, we hypothesize that these develop rapidly in the intestine and spleen of neonates as a result of exposure to antigen in the environment, or perhaps even in utero, and are induced to become memory cells with a higher state of activation compared with peripheral lymphoid tissues, even within days of birth.
Very little is known regarding the immunologic development of neonatal primate tissues, and most studies of human infants have been performed using umbilical cord blood. We previously reported that the immune system is compartmentalized in neonatal rhesus macaques.13 This report further demonstrates that the majority of lymphocytes in the blood of neonates are naive, resting cells, consistent with findings reported previously.12,20 Furthermore, the current study demonstrates that intestinal CD4+ T cells coexpress high levels of activation markers (CD25 and CD69) as well as high levels of CD95 in neonates. These results confirm that intestinal CD4+ T cells are clearly distinct from those in peripheral lymphoid tissues in terms of activation as well as naive and memory phenotyping in neonates.
Although the majority of the activated memory CD4+ T cells of neonates were observed in the intestine, there were also substantial numbers of activated memory cells in the spleen (Figure 2). These findings have also been previously reported in neonatal macaques, and it has been hypothesized that the spleen is both an inductive and effector lymphoid tissue.19 We have previously demonstrated that the spleen is also an early target of SIVmac239 infection in intravenously inoculated neonates.20 Combined, these data could explain why the spleen and intestinal lamina propria appear to be the major sites of early viral replication, amplification, and CD4+ T-cell depletion in the neonatal host.
Evidence suggests that an increased level of cellular activation may be associated with the high viral loads and rapid disease progression in the pediatric patient.26 CD25 has been previously reported to be an activation marker for T cells and may play an important role in SIV and HIV infection.23,27,28 The current study demonstrated that neonatal intestinal CD4+ T cells have much higher expression of CD25 than other tissues (Table 4). Although recent studies have shown that a subset of CD4+CD25+ T cells are associated with immunoregulation (Tregs), CD25 expression may also be associated with certain types of cell activation. We thus examined CD69 (an early marker of activation) in conjunction with CD25 in an attempt to distinguish truly activated (CD69+CD25+/−) from potential Treg (CD4+CD69−CD25+) in neonatal tissues. In this study, we define activated CD4+ T-cell subsets as CD25+CD69+ and demonstrate that there is a profound and selective loss of these cells, especially in the intestinal tract of SIV-infected neonates. As shown in Table 2, essentially all neonatal intestinal CD25+CD4+ T cells coexpressed CD69+, suggesting that these were indeed activated cells. In contrast, essentially no CD25+CD4+ T cells in the blood coexpressed CD69+. Importantly, these results demonstrate that these highly activated (CD25+CD69+) CD4+ T cells are selectively eliminated in the lamina propria of the jejunum after SIV infection (Figure 5).
We also demonstrated that this selective loss of activated memory CD4+ T cells was associated with direct viral infection of these cells. We observed that relatively large numbers of CD45RO+ cells were present in the intestinal lamina propria of all uninfected neonatal macaques examined and that these were markedly depleted after SIV infection. By 21 days after infection, marked and selective depletion of CD45RO+ cells could be detected in the intestinal lamina propria of neonates by immunohistochemistry (Figure 6). By combining in situ hybridization for SIV with a monoclonal antibody specific for CD4+CD45RO+ memory cells (OPD4), we confirmed that at least 85% of SIV-infected cells in primary infection of neonates were memory CD4+ T cells (Figure 6C). Combined, these results provide further evidence that memory CD4+ T cells are selectively infected and eliminated, particularly in the intestinal tract of SIV-infected neonates.
Although the neonates lacked significant levels of “effector memory” CD4+ T cells compared with adults, we still observed a marked, selective, and rapid loss of central memory CD4+ T cells in the neonatal intestine. By 8-color flow cytometry, we also found that central memory (CD95+CD28+) CD4+ T cells from different tissues expressed different but distinct patterns of activation markers, indicating these are a heterogeneous subset of cells. For example, few central memory CD4+ T cells in the blood (< 2%) coexpressed CD69, but about 27% of spleen and 85% of jejunum central memory cells were CD69+ (Figure 4). These data suggest that these central memory CD4+ T cells comprise different subpopulations as has been previously shown for adults.4,19
In summary, the intestinal tract of neonates harbors high levels of activated, central memory CD4+ T cells compared with blood and other peripheral lymphoid tissues. In addition, these data demonstrate that this loss of memory CD4+ T cells is due to direct viral infection rather than a result of bystander apoptosis, chronic antigenic stimulation, altered trafficking, or other mechanisms. On the basis of these results, we propose that direct viral infection and destruction of activated memory CD4+ T cells in neonates (which have larger percentages of CD4+ T cells than adults) may contribute to the higher viral loads in infants infected with HIV. Increased rates of replenishment and turnover of these cells in neonates may also be associated with the more severe disease progression in HIV-infected infants. Tissues from these and other neonatal macaques are currently being examined to quantify turnover rates of T-cell subsets for comparison with adults.
Authorship
Contribution: X.W. participated in the study design, performed the experiments, analyzed the data, prepared the figures, and contributed to writing the paper; B. Poonia and T.R. assisted with immunohistochemistry techniques; B. Pahar assisted with 8-color flow cytometry; X.A. assisted with confocal microscopy; A.A.L. participated in study design and writing the paper; R.S.V. designed the study and assisted with data analysis and in writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald S. Veazey, Tulane National Primate Research Center, 18703 Three Rivers Rd, Covington, LA 70433; e-mail: rveazey@tulane.edu.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Linda Green, Janell LeBlanc, Maryjane Dodd, and Kelsi Rasmussen for technical assistance. We thank Julie Bruhn, Calvin Lanclos, and Ed Benes for flow cytometry support.
This work was supported by the National Institutes of Health (grants AI062410, AI49080, RR00164, RR016930, RR018397, RR019628, RR013466, RR012112, and RR05169).