A growing body of evidence indicates an intimate link between the mesenchymal tissue of the thymus and thymic epithelial cell proliferation, which provides a new regulatory system of niche formation and, consequently, proper T lymphocyte progenitor differentiation into functional T cells.
In the last few years, several studies have focused on mesenchyme contribution to tissue development and function. As in the case of astrocytes in central nervous system development, there has been increasing attention to how neural crest–derived thymic stromal cells influence the intrathymic niche structure and function. Thymic niches promote and regulate T lymphocyte development from undifferentiated thymocytes. There is evidence suggesting that mesenchyme cells do not merely work as structural components, but their functional role has not been completely elucidated.
It has been clearly demonstrated that mesenchymal cells influence thymic epithelial cell growth, presumably through the release of soluble factors, such as those belonging to the fibroblast growth factor (FGF) family, into the extracellular matrix. In fact, the addition of recombinant FGF7 and FGF10 rescues proliferation of thymic epithelial cells when cultured in the absence of mesenchyme.1
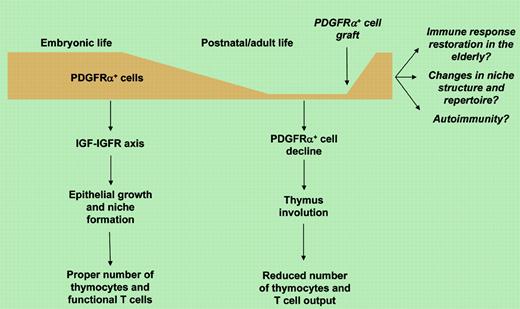
In this issue of Blood, Jenkinson and colleagues go deep into analyzing the stromal cell's role in building the thymus. They report that the nonepithelial platelet-derived growth factor receptor-α–positive (PDGFRα+) thymic fetal mesenchymal cells promote the growth of thymic epithelial cells and the development of intrathymic niches, and regulate thymus size. The authors demonstrate that such fetal thymic mesenchyme has specialized properties in promoting epithelial growth that are not shared with adult mesenchymal tissue. Surprisingly, this mesenchyme specialization is unrelated to the previously described release of FGF7 and FGF10 and appears to be due to the production of insulin-like growth factors (IGFs) and the consequent growth stimulation of IGFR+ thymic epithelial cells. This novel mechanism indicates that different signals regulate epithelial growth and intrathymic niche formation during embryonic and adult life. Based on these observations, the authors suggest that mesenchyme restoration may represent a potential therapeutic strategy for reconstitution from drug-induced or elderly immune deficiency.
The gradual loss of mesenchymal tissue in the developing thymus underlines the well-known progressive thymic regression during postnatal and adult life and raises several issues. Given that few PDGFRα+ mesenchymal cells are also present in adult thymus (see figure), do they regulate T-cell development, and if so, through which mechanisms? Can mesenchymal cell graft in the adult stimulate rebuilding of thymic niches and cause T lymphocyte repertoire changes? Is there any link between the onset of autoimmune disease and potential dysregulation in thymic mesenchyme in the adult thymus? This last question arises in consideration of the relevant role that epithelial cells play in regulating T-cell selection against autoantigens.2,3
While providing a rigorous anatomical and temporal dissection of thymus development, Jenkinson and colleagues add another sturdy brick into the mechanism of thymus development in the quest for new immune-modulating therapies.
The author declares no conflicting financial interests. ▪