Research on leukocyte extravasation has used a vivid and elegant heuristic scheme, termed the multistep paradigm,1 which sought to characterize discrete molecular entities as regulators of leukocyte-endothelial interaction,2 which in turn governed leukocyte trafficking. New work from Schreiber and colleagues using a novel transwell-based system yields additional and surprising insights.
Leukocyte trafficking underlies inflammation and immunity. The remarkable specificity with which, for example, naive lymphocytes recognize lymph node high endothelial venules (HEVs) has excited wonder and curiosity. Researchers, studying this amazingly selective and rapid information transfer between static vascular endothelial cells and rapidly-moving leukocytes, fastened on the discrete steps of leukocyte-endothelial interaction as a promising starting point. Early observations used flow chambers, in which leukocytes were perfused at physiologic flow rates past adhesive surfaces reconstituted with recombinant endothelial adhesion molecules, and showed that initial leukocyte-endothelial tethers depended mainly on selectins and their carbohydrate ligands. Selectins could be expressed either by leukocytes or endothelium, with counterreceptors on opposing cell membranes. Tethered leukocytes, under fluid shear forces, rolled across the endothelial surface, either to detach and re-enter the bloodstream or to undergo activation and subsequent arrest. Activating signals were delivered through G-protein–coupled receptors (GPCRs) upon encounter with ligands, including chemokines, leukotrienes, or platelet-activating factor (see figure). Activation through the GPCR induced clustering and conformational change of leukointegrins, leading to high-affinity/avidity binding to endothelial adhesion counterreceptors, with subsequent leukocyte arrest, viewed as the immediate prelude to extravasation.
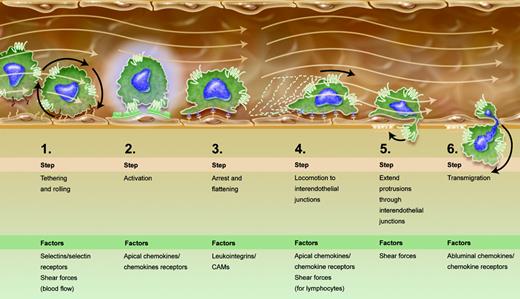
The enriched multistep paradigm for leukocyte extravasation. Progressive stages of leukocyte-endothelial interaction lead to diapedesis. Given new findings by Schreiber et al and other recent publications,4,5 steps 4 and 5 were added to the canonical paradigm.1 Apical and abluminal chemokines are shown to exert distinct functions. Shear forces of flowing blood are integrated into the scheme as promoting certain stages of the process, rather than being sheerly disruptive. Illustration by A. Y. Chen.
The enriched multistep paradigm for leukocyte extravasation. Progressive stages of leukocyte-endothelial interaction lead to diapedesis. Given new findings by Schreiber et al and other recent publications,4,5 steps 4 and 5 were added to the canonical paradigm.1 Apical and abluminal chemokines are shown to exert distinct functions. Shear forces of flowing blood are integrated into the scheme as promoting certain stages of the process, rather than being sheerly disruptive. Illustration by A. Y. Chen.
As is so often the case, Schreiber et al's use of new technology led to new knowledge. Previous work used Boyden chambers, which did not incorporate flow, but could shed light on chemotactic responses. Intravital microscopy, used to study adhesion molecules, endothelial and leukocyte activation, and roles of GPCRs,3 was difficult to manipulate.
Recent studies used parallel plate flow chambers to show that shear stress signals promote transendothelial migration of lymphocytes over endothelial monolayers reconstituted with apically adsorbed chemokines,4 but could not address the relative contributions of apical and abluminal chemokines. Schreiber and colleagues used a modified Boyden chamber to dissect these questions, through application of physiologic shear forces and real-time videomicroscopy. The results extended prior findings about chemokines associated with the luminal endothelial surface (termed apical chemokines by Schreiber et al). Also designated “arrest” chemokines, these chemokines have long been considered essential for integrin activation and leukocyte arrest. Recent findings also show that apical chemokine exposure drives postarrest lymphocyte locomotion along the endothelial surface, in search of interendothelial junctions, the sites of lymphocyte transendothelial migration in vitro.4 The importance of locomotion in leukocyte diapedesis was also underscored by recent work conducted under shear-free conditions,5 suggesting that locomotion needs to be included in the stages enumerated in the multistep paradigm (see figure). Schreiber et al also underline roles of shear forces in transendothelial migration. Most reviews treat fluid shear forces as disruptors of leukocyte-endothelial interactions, and emphasize how adhesion molecules overcome this distraction. The current report shows that shear forces are necessary for the establishment and stability of lymphocyte protrusions into interendothelial junctions and proposes that these protrusions may concentrate chemokine receptors to better sense abluminal chemokines and thereby drive transendothelial migration (see figure).
It is now mandatory to include locomotion as an obligate step taken, after arrest, by leukocytes that extravasate by paracellular rather than transcellular routes. Once locomoting leukocytes reach interendothelial junctions, they then need to send protrusions enriched with chemokine receptors to sense the abluminal environment for chemotactic cues. Shear forces must be incorporated into the list of factors that regulate leukocyte extravasation since they critically regulate both apical locomotion and protrusions into the basal endothelial compartments. It is indeed tempting to consider that differential strengths of shear forces in varied vascular beds as well as apical and abluminal chemokines help to define the specificity of leukocyte trafficking. This work by Schreiber et al also implies functional distinctions among 3 pools of chemokines to which leukocytes are exposed: soluble chemokines in circulation with unknown functions that may include receptor down-regulation; apical chemokines presented on the endothelium that generate signals for arrest and locomotion under flow; and subendothelial chemokines that drive transendothelial migration and basal locomotion. The cumulative impact of these new data may fall short of a paradigm shift but surely represents a substantial paradigm enrichment.
The authors declare no competing financial interests. ▪