Abstract
The critical role of Bruton tyrosine kinase (Btk) in B cells has been documented by the block of B-cell development in X-linked agammaglobulinemia (XLA). Less is known about Btk function in myeloid cells. Several pieces of evidence indicate that Btk is a component of Toll-like receptor (TLR) signaling. We analyzed whether Btk deficiency in XLA is associated with an impaired dendritic cell (DC) compartment or defective TLR signaling. We analyzed the expression of TLRs 1 to 9 on myeloid DCs generated from XLA patients and evaluated their response to activation by specific TLR agonists. We show that XLA patients have normal numbers of circulating DCs. Btk-deficient DCs have no defect in response to stimulation of TLRs 1/2, 2/6, 3, 4, and 5 but display a profound impairment of IL-6 and TNF-α production in response to stimulation by TLR-8 cognate agonist, ssRNA. These findings may provide an explanation for the susceptibility to enteroviral infections in XLA patients.
Introduction
Bruton tyrosine kinase (Btk) is a member of the Tec family of protein tyrosine kinases, expressed in all hematopoetic cells except T cells and natural killer (NK) cells. The critical role of Btk signaling in the development, activation, and survival of B cells has been documented by the block of B-cell development and absence of mature B cells in the peripheral blood of patients with X-linked agammaglobulinemia (XLA), a primary immunodeficiency caused by loss-of-function mutations in Btk.1
Less is known about the function of Btk in the myeloid cells. Recent studies have shown multiple defects in the development and function of myeloid cells in xid mice, a murine counterpart of XLA.2 In accordance with these reports, peripheral blood mononuclear cells (PBMCs) from XLA patients were shown to produce reduced amounts of TNF-α in response to LPS stimulation.3 Several pieces of evidence indicate that Btk is a component of Toll-like receptor (TLR) signaling pathways. Mammalian TLRs are crucial for the recognition of pathogen-associated molecular patterns (PAMPs), structures unique to microorganisms and shared among infectious agents. At least 10 TLRs have been identified in humans and are expressed predominantly on macrophages, dendritic cells (DCs), neutrophils, and monocytes. All TLRs contain extracellular leucine-rich repeat domains and a cytoplasmic signaling domain known as the Toll–interleukin-1 receptor (TIR) domain that mediates signaling through TLRs. Recognition of PAMPs by corresponding TLRs leads to the activation of antigen-presenting cells, mainly DCs, and represents a crucial step in the initiation of innate and adaptive immune responses. TLR-activated immature DCs enhance expression of surface costimulatory and antigen presentation–associated molecules, produce proinflammatory and Th1 polarizing cytokines, migrate to the secondary lymphoid organs, and activate antigen-specific T cells.4 Moreover, TLR signaling has recently been shown to be important for DC differentiation. Btk has been shown to interact with TIR domains of TLRs 4, 6, 8, and 9 and was also found to associate with other components of TLR signaling. LPS, TLR-4 agonist, induces phosphorylation of Btk and activates its kinase activity.5 Given the importance of TLR signaling for the activation of DCs and for the activation of innate and adaptive immune response, we decided to address the issue of whether Btk deficiency in XLA patients is associated with an impaired DC compartment or defective TLR signaling.
Patients, materials, and methods
Six male patients diagnosed with XLA, according to the World Health Organization (WHO) classification, and 15 healthy controls were included in this study after informed consent was obtained. The study was approved by the investigational review board (IRB) of 2nd Medical School, Charles University, Prague, Czech Republic. Btk mutations were identified in all patients by direct sequencing of cDNA samples as described. All patients were receiving regular intravenous immunoglobulin (IVIG) replacement therapy at 3- to 4-week intervals and were free of any serious infections at the time of blood sampling. Heparinized blood samples were collected prior to the infusion of IVIG. Monocyte-derived DCs from patients' blood and from control groups were generated as described previously.6,7 Expression of TLRs 1 to 9 on in vitro–generated DCs was analyzed by real-time reverse transcriptase–polymerase chain reaction (RT-PCR) (for details, contact the corresponding author).
Immature DCs were stimulated by TLR-specific agonists, and their phenotypic and functional characteristics were studied as described previously.8 TLR agonists were used in the concentrations that induced significant cytokine production in control DCs without impairing their viability (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article).
For in vivo DC quantification, peripheral blood was stained by FITC-conjugated lineage cocktail monoclonal antibodies (mAbs) (CD3, CD14, CD16, CD19, and CD56), PE-CD123, PC5–HLA-DR, and APC-CD11c mAbs. We defined myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) as lineage-negative/HLA-DR+/CD11c+ and lineage-negative/HLA-DR+/CD123+ cells, respectively. The absolute number of mDCs and pDCs was calculated from the white blood count multiplied by the proportion of each subset within the population of white blood cells. DC subsets were analyzed on FACS Aria (Becton Dickinson, Prague, Czech Republic).
The Mann-Whitney test was used for statistical analysis, and P below .05 was considered significant.
Results and discussion
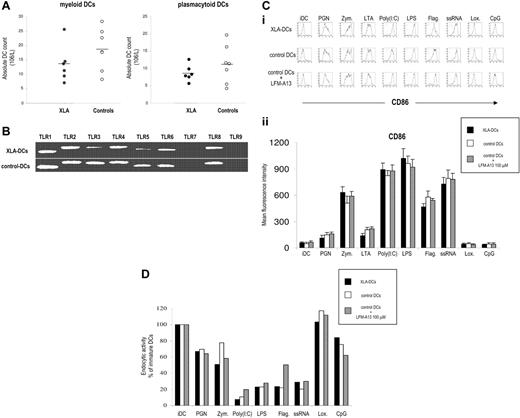
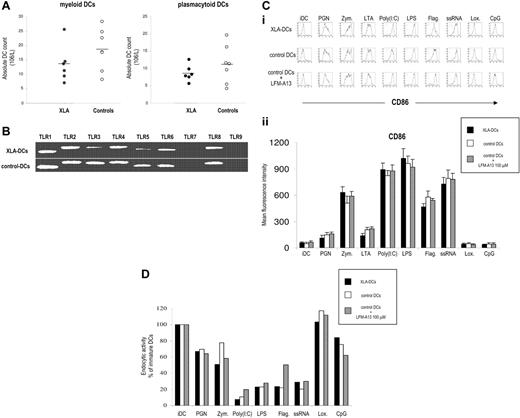
TLR signaling is important for the differentiation of DCs from their precursors.9 In the first set of experiments, we analyzed the frequency and absolute numbers of myeloid and plasmacytoid DCs subsets in peripheral blood of XLA patients. Although there was a tendency toward lower numbers of DCs in XLA patients, the difference was not statistically significant for any subset (Figure 1A).
Subsets of mDCs and pDCs in XLA patients. (A) Peripheral blood DC subsets in XLA patients and healthy controls. Myeloid DCs (mDCs) were identified as lineage-negative/HLA-DR+/CD11c+ cells and plasmacytoid DCs (pDCs) as lineage-negative/HLA-DR+/CD123+ cells. Absolute numbers of circulating DC subsets in peripheral blood are shown. Points correspond to individual patients; horizontal bars represent the mean value for the group. (B) Expression of TLRs 1 to 9 on immature DCs from XLA patients and healthy blood donors analyzed by RT-PCR. Both XLA-DC and control DCs expressed TLRs 1 to 6 and 8, a profile characteristic of myeloid DCs. As expected, we did not detect TLR-7 and TLR-9, molecules typically expressed on plasmacytoid DCs. Representative results of 1 of 5 independent experiments are shown. (C) Characteristics of DCs in XLA patients. (i) CD86 expression (thick line) versus isotype control (thin line) is shown. Representative results of 1 of 10 experiments are shown. (ii) Summary of CD86 expression on XLA-DCs, control DCs, and control DCs plus LFM-A13 after activation with TLR agonists. Average of mean fluorescent intensity values plus SDs for 5 different XLA patients and healthy controls is shown. (D) Endocytic activity of XLA-DCs and DCs from healthy donors after stimulation with TLR agonists.
Subsets of mDCs and pDCs in XLA patients. (A) Peripheral blood DC subsets in XLA patients and healthy controls. Myeloid DCs (mDCs) were identified as lineage-negative/HLA-DR+/CD11c+ cells and plasmacytoid DCs (pDCs) as lineage-negative/HLA-DR+/CD123+ cells. Absolute numbers of circulating DC subsets in peripheral blood are shown. Points correspond to individual patients; horizontal bars represent the mean value for the group. (B) Expression of TLRs 1 to 9 on immature DCs from XLA patients and healthy blood donors analyzed by RT-PCR. Both XLA-DC and control DCs expressed TLRs 1 to 6 and 8, a profile characteristic of myeloid DCs. As expected, we did not detect TLR-7 and TLR-9, molecules typically expressed on plasmacytoid DCs. Representative results of 1 of 5 independent experiments are shown. (C) Characteristics of DCs in XLA patients. (i) CD86 expression (thick line) versus isotype control (thin line) is shown. Representative results of 1 of 10 experiments are shown. (ii) Summary of CD86 expression on XLA-DCs, control DCs, and control DCs plus LFM-A13 after activation with TLR agonists. Average of mean fluorescent intensity values plus SDs for 5 different XLA patients and healthy controls is shown. (D) Endocytic activity of XLA-DCs and DCs from healthy donors after stimulation with TLR agonists.
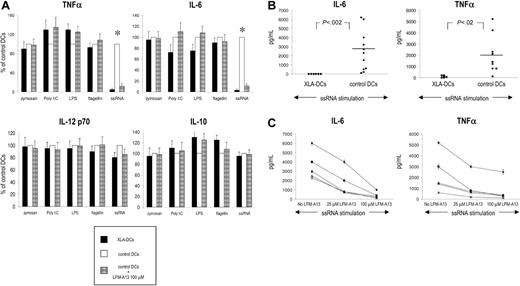
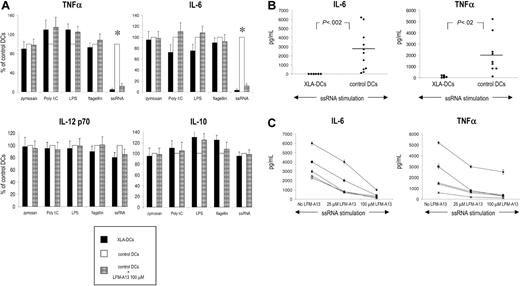
We then generated immature monocyte-derived DCs from monocytes of XLA patients (XLA-DCs) to evaluate their ability to be activated in response to TLR stimulation. Monocytes from XLA patients differentiated normally in immature DCs, and there was no difference in the yield, morphology, survival, or phenotype between XLA-DCs and DCs from healthy donors (data not shown). Both XLA-DCs and control DCs expressed TLRs 1 to 6 and 8, a profile characteristic of myeloid DCs. As expected, we did not detect TLR-7 and TLR-9, molecules typically expressed on plasmacytoid DCs (Figure 1B). Activation of XLA-DCs, control DCs, or control DCs in the presence of LFM-A13, a Btk inhibitor, with TLR agonists led to similar maturation-related changes in surface molecule expression (ie, up-regulation of CD80, CD83, CD86, and HLA classes I and II) (Figure 1C and data not shown). Decrease of endocytosis is another hallmark of DC maturation, and stimulation of TLRs led to the significant decline in the ability of XLA-DCs and control DCs to endocytose FITC-labeled dextran (Figure 1D). Similarly, XLA-DCs and control DCs were comparable in their capacity to induce CD4 T-cell proliferation when used as stimulators in allogeneic mixed leukocyte reactions (data not shown). We next evaluated the production of proinflammatory and Th1 polarizing cytokines after TLR stimulation. Stimulation of TLRs 1 to 6 induced comparable IL-6, TNF-α, IL-10, and IL-12 production by XLA-DCs, control DCs, and DCs preincubated with LFM-A13 (Figure 2A). However, stimulation with TLR-8 agonist, ssRNA, failed to induce any IL-6 and TNF-α production by XLA-DCs, while control DCs produced high levels of both cytokines after ssRNA stimulation (P < .002 and P < .02 for IL-6 and TNF-α, respectively; Figure 2A-B). To determine whether this dramatic defect in cytokine production could be attributed to Btk deficiency, control DCs were pretreated with LFM-A13 before ssRNA stimulation. Preincubation of control DCs with LFM-A13 significantly decreased IL-6 and TNF-α production in a dose-dependent manner without affecting the viability of DCs (Figure 2C). XLA-DCs thus have impaired capacity to produce IL-6 and TNF-α in response to TLR-8 stimulation.
Cytokine production by XLA-DCs and DCs from healthy controls after stimulation with TLR agonists. (A) Relative production of TNFα, IL-6, IL-12 p70, and IL-10 by XLA-DCs, control DCs, and control DCs preincubated with 100 μM LFM-A13 after stimulation with TLR agonists. Data are expressed as relative production as compared with control DCs. Bars show means/SD of 4 different patients and healthy controls. *P value for comparison with control DCs; P < .05. (B) IL-6 and TNF- α production by XLA-DCs and DCs generated from healthy controls after ssRNA stimulation. Dots represent individual patients; horizontal bars, means. The Mann-Whitney test was used for statistical analysis. (C) The effect of Btk inhibitor LFM-A13 (at 2 different concentrations) on the production of TNF-α and IL-6 by DCs generated from 5 healthy blood donors. Representative results of 5 independent experiments are shown.
Cytokine production by XLA-DCs and DCs from healthy controls after stimulation with TLR agonists. (A) Relative production of TNFα, IL-6, IL-12 p70, and IL-10 by XLA-DCs, control DCs, and control DCs preincubated with 100 μM LFM-A13 after stimulation with TLR agonists. Data are expressed as relative production as compared with control DCs. Bars show means/SD of 4 different patients and healthy controls. *P value for comparison with control DCs; P < .05. (B) IL-6 and TNF- α production by XLA-DCs and DCs generated from healthy controls after ssRNA stimulation. Dots represent individual patients; horizontal bars, means. The Mann-Whitney test was used for statistical analysis. (C) The effect of Btk inhibitor LFM-A13 (at 2 different concentrations) on the production of TNF-α and IL-6 by DCs generated from 5 healthy blood donors. Representative results of 5 independent experiments are shown.
The role of Btk in TLR signaling pathways has been recently suggested. Btk is phosphorylated in response to the stimulation of TLR-4 by LPS and associates with TIR domains of TLRs 4, 6, 8, and 9.5 A very recent report has identified Mal as the downstream target of Btk tyrosine kinase function in myeloid cells.10 Here we show that absence of Btk in XLA has no effect on the frequency and phenotypic characteristics of circulating DCs subsets. Moreover, XLA-DCs have TLR expression patterns similar to control DCs, and their activation by specific agonists for TLRs 1/2, 2/6, 3, 4, and 5 leads to analogous phenotypic and functional changes as in control DCs. In a pioneer study, Gagliardi et al11 evaluated the effect of LPS on some aspects on DC maturation and did not detect any differences from control DCs. Absence of any detectable defect in LPS-mediated signaling has also been recently documented in monocytes from XLA patients.12 However, in contrast with these reports and with our own findings, 2 recent reports showed a slight decrease in IL-1 and TNF-α production by XLA monocytes and macrophages stimulated with LPS and Palm-Cys, TLR-4 and TLR-2 agonists, respectively.3,13 We did not confirm the impaired DC function after TLR-4 binding. However, we detected a profound defect in IL-6 and TNF-α production by XLA-DCs in response to TLR-8 stimulation by its cognate agonist, ssRNA.14,15 Selective impairment of IL-6 and TNF-α production suggests existence of different intracellular signaling pathways in response to ssRNA, as recently reported by Kanneganti et al.16 Finding of an impaired TLR-8 response is interesting with respect to the clinical presentations of XLA patients. Although periodic infusions of IVIG lead to satisfactory levels of circulating antibodies, XLA patients frequently suffer from chronic and potentially very severe and fatal enteroviral infections (ssRNA viruses).17 Given the crucial importance of pathogen recognition for the homeostasis and survival, mammals have evolved multiple and redundant sensory mechanisms. This redundancy can be reflected at the level of individual TLRs (one TLR can be linked to multiple signaling pathways via different adaptor proteins). More importantly, pathogens are usually recognized by multiple pattern recognition receptors, including TLRs.18 Such redundancy limits the risk of systemic life-threatening infections by large groups of pathogens. However, it is conceivable that while accessory mechanisms of pathogen recognition provide sufficient protection against most infections, a subtle defect in the signaling pathway can account for an increased frequency of infections caused by a particular pathogen.19 Enteroviral infections are in fact the major cause of mortality in XLA patients. Inappropriate and insufficient recognition of viral ssRNA by Btk-deficient DCs might thus contribute to the susceptibility of XLA patients to enteroviral infections.
Authorship
Contribution: K.S., R.H., and D.R. performed research; J.L. coordinated patients' samples; J.B. designed research; and A.S. and R.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A.S. and R.S. contributed equally to this study.
Correspondence: Radek Špíšek, Institute of Immunology, Charles University, Second Medical School, V Uvalu 84, Prague 5, Czech Republic; e-mail: rspisek@rockefeller.edu.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank T. Kalina for thoughtful comments and technical assistance with flow cytometry experiments. This study was supported by project MSM 0021620812 from The Czech Ministry of Education, EU 6th Framework Program Allostem LSHB-CT-2004-503319, and grant GAUK 62/2005 provided by the internal grant agency of Charles Agency (K.S.).