What is a hemangioblast? The term can be traced to the last century when putative bipotential cells in the blood islands of vertebrate embryos displayed the ability to give rise to both the endothelial and hematopoietic lineages.
While studies in the chick, frog, and zebrafish have all supported the concept that such a cell exists, most of the evidence for the hemangioblast has been accrued in experiments studying murine embryogenesis and murine embryonic stem cell–derived embryoid body (EB) differentiation. In particular, the development of a clonal assay that permitted isolation of murine blast colony–forming cells (BL-CFCs) proved most useful in defining the presence of hemangioblasts in vitro and in vivo.1,2 Murine embryonic stem cell (mESC) differentiation studies document the emergence of the BL-CFC prior to development of the primitive or definitive hematopoietic and endothelial lineages, and the pattern of hematopoietic and endothelial cell emergence from the BL-CFC and mESC-derived EB mirrors the temporal emergence of these cells in the murine embryo.3 The development of human embryonic stem cells (hESCs) has generated considerable enthusiasm that these cells may serve as a reagent for producing large numbers of blood cells for regenerative or replacement therapies in human subjects. Most comparative studies of mESCs and hESCs have pointed out the differences in the maintenance of culture conditions, morphology of the ESC colonies, cell surface antigen expression, growth factor requirements to maintain ESC self-renewal, and conditions required to promote hematopoietic and endothelial cell differentiation.
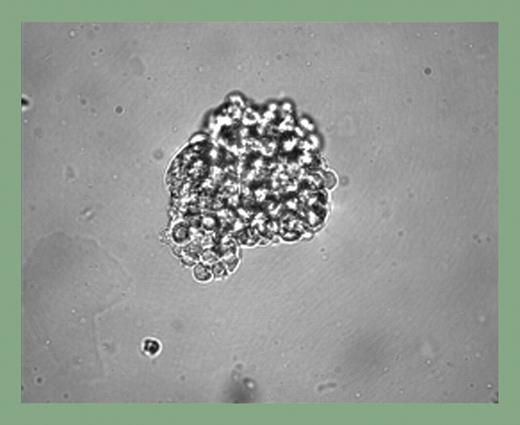
The study by Kennedy and colleagues presents novel insights into hemangioblast development from hESCs and suggests that significant similarities may exist with the mESC system. Development of a novel human BL-CFC assay, serum-free culture differentiation conditions, and elucidation of the specific requirements for hemangioblast development are all significant advances contained in this single report. Bone morphogenetic protein-4 was found to be an essential factor in mesoderm induction and hemangioblast development, and BL-CFC (see figure) development from the hemangioblast required basic fibroblast growth factor and vascular endothelial growth factor in a fashion similar to mESC differentiation conditions. Of interest, 2 broad classes of hemangioblasts were identified: those that generated primitive erythrocytes exclusively and those that gave rise to primitive erythrocytes and macrophages. The first site of human embryonic blood cell production occurs in the yolk sac between days 16 and 19 of gestation, and the blood islands observed are composed of endothelial cells, primitive erythroid cells, and rare macrophages. Thus, the primitive erythroid and macrophage potential of the in vitro–cultured hESC-derived hemangioblast is consistent with the known blood cells present in the early human embryo.
Blast cell colony development from human EBs. See the complete figure in the article beginning on page 2679.
Blast cell colony development from human EBs. See the complete figure in the article beginning on page 2679.
While this paper represents a significant advance for the field, many important questions remain to be addressed. Will definitive progenitor cells, perhaps even lymphoid progeny, emerge from the hESC-derived BL-CFC with appropriate induction conditions? Will the availability of the BL-CFC assay permit identification of novel cell surface antigens that mark the hemangioblast and allow for isolation and detailed study of this rare progenitor? Is the hemangioblast sustained into adulthood?4 This cell will surely remain an interesting and controversial progenitor in both mice and men.
The author declares no competing financial interests. ▪