Abstract
Celastrol, a quinone methide triterpene derived from the medicinal plant Tripterygium wilfordii, has been used to treat chronic inflammatory and autoimmune diseases, but its mechanism is not well understood. Therefore, we investigated the effects of celastrol on cellular responses activated by TNF, a potent proinflammatory cytokine. Celastrol potentiated the apoptosis induced by TNF and chemotherapeutic agents and inhibited invasion, both regulated by NF-κB activation. We found that TNF induced the expression of gene products involved in antiapoptosis (IAP1, IAP2, Bcl-2, Bcl-XL, c-FLIP, and survivin), proliferation (cyclin D1 and COX-2), invasion (MMP-9), and angiogenesis (VEGF) and that celastrol treatment suppressed their expression. Because these gene products are regulated by NF-κB, we postulated that celastrol mediates its effects by modulating the NF-κB pathway. We found that celastrol suppressed both inducible and constitutive NF-κB activation. Celastrol was found to inhibit the TNF-induced activation of IκBα kinase, IκBα phosphorylation, IκBα degradation, p65 nuclear translocation and phosphorylation, and NF-κB–mediated reporter gene expression. Recent studies indicate that TNF-induced IKK activation requires activation of TAK1, and we indeed found that celastrol inhibited the TAK1-induced NF-κB activation. Overall, our results suggest that celastrol potentiates TNF-induced apoptosis and inhibits invasion through suppression of the NF-κB pathway.
Introduction
Modern targeted therapies for most chronic illnesses, including cancer, have been mostly unsuccessful because of their ineffectiveness, lack of safety, and cost. Although monotherapy was advocated at one time, recent experience indicates that agents that target multiple pathways have more potential in cancer treatment. This understanding has led to combination therapy. Although traditional therapies have been around for thousands of years, their active components and their mechanisms of action remain undefined. Identification of an active chemical entity and its molecular targets can lead to rediscovery of the clinical potential of such therapies, as in the case of paclitaxel, derived from Taxus (yew) species during a random screening of US Department of Agriculture collection of plants.1
Celastrol, also known as tripterine, is one such compound that was originally identified from traditional Chinese medicine (“God of Thunder Vine”) almost 3 decades ago and used for the treatment of cancer and other inflammatory diseases.2 Celastrol, a triterpenoid from the Celastracae family and extracted from the plant Tripterygium wilfordii,3 has attracted interest, especially for its potential anti-inflammatory effects.4 The in vivo anti-inflammatory effects of this triterpene have been demonstrated in animal models of collagen-induced arthritis,5 Alzheimer disease,6 asthma,7 systemic lupus erythematosus,8,9 and rheumatoid arthritis.10 Celastrol is also known to inhibit the proliferation of a variety of tumor cells, including those from leukemia,11 gliomas,12 and prostate cancer.13 The ability of celastrol to modulate the expression of proinflammatory cytokines,3,14,15 MHC-II antigen,6 inducible nitric oxide (NO) synthase (iNOS),16 adhesion molecules in endothelial cells,17 proteasome activity,13 topoisomerase II,11 potassium channels,18 and heat shock response19 has been reported. However, the molecular mechanism underlying the anti-inflammatory and antiproliferative effects of celastrol is not yet fully understood.
Because of the role of the transcription factor nuclear factor (NF)-κB in inflammation and cellular proliferation,20-23 we speculated that celastrol mediates its effects by modulating this pathway. Our results demonstrate that celastrol potentiates TNF-induced apoptosis and inhibits invasion by down-regulating NF-κB–regulated antiapoptotic gene products and the NF-κB activation pathway.
Materials and methods
Reagents and antibodies
A 50-mM solution of celastrol (Cayman Chemicals, Ann Arbor, MI) was prepared in 100% dimethyl sulfoxide, stored as small aliquots at −20°C, and then diluted as needed in cell-culture medium. Bacteria-derived recombinant human TNF, purified to homogeneity with a specific activity of 5 × 107 U/mg, was kindly provided by Genentech (South San Francisco, CA). Paclitaxel, doxorubicin, and β-actin antibody were obtained from Aldrich-Sigma (St Louis, MO). Antibodies against p65, p50, IκBα, cyclin D1, MMP-9, PARP, IAP1, IAP2, Bcl-2, and Bcl-XL were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–COX-2 antibody was obtained from BD Biosciences (San Diego, CA). Phospho-specific anti-IκBα (Ser32/36) and phospho-specific anti-p65 (Ser536) antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti–IKK-α, anti–IKK-β, and c-FLIP antibodies were kindly provided by Imgenex (San Diego, CA). Antivascular endothelial growth factor (VEGF) was purchased from NeoMarkers (Fremont, CA). FuGENE 6 was purchased from Roche (Nutley, NJ). Survivin antibody was purchased from R&D Systems (Minneapolis, MN).
Cell lines
Human myeloid KBM-5 cells, human lung adenocarcinoma H1299 cells, human multiple myeloma U266 cells, human bladder cancer 253JBV cells, and human embryonic kidney A293 cells were obtained from American Type Culture Collection (Manassas, VA). KBM-5 cells were cultured in IMDM supplemented with 15% fetal bovine serum (FBS). H1299 cells and U266 cells were cultured in RPMI 1640 medium, and A293 cells were cultured in DMEM supplemented with 10% FBS. 253JBV cells were cultured in DMEM containing 10% FBS, nonessential amino acids, pyruvate, glutamine, and vitamins. All media were also supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin.
Cytotoxicity assay
Cytotoxicity was assayed by the modified tetrazolium salt 3-(4-5-dimethylthiozol-2-yl)2-5-diphenyl-tetrazolium bromide (MTT) assay as described previously.24
PARP cleavage assay
To detect cleavage of PARP, we prepared whole-cell extracts by subjecting celastrol-treated cells to lysis in lysis buffer (20 mM Tris, pH 7.4, 250 mM NaCl, 2 mM EDTA, pH 8.0, 0.1% NP-40, 0.01 μg/mL aprotinin, 0.005 μg/mL leupeptin, 0.4 mM PMSF, and 4 mM sodium orthovanadate). Lysates were spun at 14 000 rpm for 10 minutes to remove insoluble material, resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and probed with PARP antibody.
Live/dead assay
To measure apoptosis, we used the Live/Dead Cell Viability assay (Molecular Probes, Eugene, OR), which determines intracellular esterase activity and plasma membrane integrity. This assay uses calcein, a polyanionic dye, which is retained within live cells and provides green fluorescence.25 It also uses the ethidium monomer dye (red fluorescence), which can enter cells only through damaged membranes and then bind to nucleic acids but is excluded by the intact plasma membrane of live cells. Briefly, 1 × 106 cells were incubated with 2.5 μM celastrol for 6 hours and then treated with 1 nM TNF for 16 hours at 37°C. Cells were stained with the live/dead reagent (5 μM ethidium homodimer, 5 μM calcein-am) and then incubated at 37°C for 30 minutes. Cells mounted with Mounting Media (Sigma-Aldrich) were analyzed under a fluorescence microscope (Lapshot-2, Nikon, Tokyo, Japan) equipped with a CFWN 10×/1.5 oil-immersion objective lens and a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX). Images were acquired with MetaMorph 4.6.5 software (Universal Imaging, Downingtown, PA).
TUNEL assay
We also assayed apoptosis by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) method, which examines DNA strand breaks during apoptosis with an in situ cell death detection reagent (Roche Diagnostics, Mannheim, Germany). This assay was performed as described previously.25
Invasion assay
The membrane invasion culture system was used to assess cell invasion because invasion through the extracellular matrix is a crucial step in tumor metastasis. The BD BioCoat Tumor Invasion system is a chamber that has a light-tight polyethylene terephthalate membrane with 8-μm diameter pores and is coated with a reconstituted basement membrane gel (BD Biosciences). A total of (2.5 × 104) H1299 cells were suspended in serum-free medium and seeded into the upper wells. After incubation overnight, cells were treated with 2.5 μM celastrol for 6 hours and then stimulated with 1 nM TNF for a further 24 hours in the presence of 1% FBS and celastrol. Cells that invaded through the Matrigel (those that migrated to the lower chamber during incubation) were stained with 4 μg/mL calcein-am (Molecular Probes) in PBS for 30 minutes at 37°C and scanned for fluorescence with a Victor 3 multiplate reader (Perkin Elmer Life and Analytical Sciences, Boston, MA); fluorescent cells were counted.
Electrophoretic mobility shift assay
To determine NF-κB activation by TNF, we performed an electrophoretic mobility shift assay (EMSA) essentially as described previously.26 Briefly, nuclear extracts prepared from TNF-treated cells (2 × 106/mL) were incubated with 32 P-end-labeled 45-mer double-stranded oligonucleotide (15 μg protein with 16 fmol DNA) from the HIV long terminal repeat, 5′-TTGTTACAA GGGACTTTC CGCTG GGGACTTTC CAGGGAGGCGTGG-3′ (boldface indicates NF-κB binding sites), for 30 minutes at 37°C. The DNA-protein complex formed was separated from free oligonucleotide on 6.6% native polyacrylamide gels. A double-stranded mutated oligonucleotide, 5′-TTGTTACAA CTCACTTTC CGCTG CTCACTTTC CAGGGAGGCGTGG-3′, was used to examine binding specificity of NF-κB to the DNA. The binding specificity was also examined by competition with the unlabeled oligonucleotide. For supershift assays, nuclear extracts prepared from TNF-treated cells were incubated with antibodies against either the p50 or the p65 subunit of NF-κB for 30 minutes at 37°C before the complex was analyzed by EMSA. Preimmune serum (PIS) was included as a negative control. The dried gels were visualized with a Storm 820, and radioactive bands were quantified using Imagequant software (Amersham Pharmacia Biotechnology, Piscataway, NJ).
Western blot analysis
To determine the effect of celastrol on TNF-dependent IκBα phosphorylation and IκBα degradation, we prepared cytoplasmic extracts as previously described27 and probed them with specific antibodies against IκBα and phosphorylated IκBα. To determine the expression of cyclin D1, COX-2, MMP-9, IAP1, IAP2, Bcl-2, Bcl-XL, VEGF, c-FLIP, and survivin whole-cell extracts from TNF and celastrol plus TNF-treated cells were prepared and 30 μg protein was resolved on SDS-PAGE and probed with specific antibodies according to the manufacturer's recommended protocol. The blots were washed, exposed to HRP-conjugated secondary antibodies for 1 hour, and finally detected by enhanced chemiluminescence (ECL) reagent (Amersham Pharmacia Biotechnology). The bands were quantified with a Personal Densitometer Scan v1.30 using Imagequant software version 3.3 (Molecular Dynamics, Piscataway, NJ).
IKK assay
To determine the effect of celastrol on TNF-induced IKK activation, we analyzed IKK by a method essentially as described previously.28 Briefly, the IKK complex from whole-cell extracts was precipitated with IKKα and IKKβ and treated with protein A/G-agarose beads (Pierce Chemical, Rockford, IL). After 2 hours, the beads were washed with lysis buffer and resuspended in a kinase assay mixture containing 50 mM HEPES (pH 7.4), 20 mM MgCl2, 2 mM dithiothreitol, 20 μCi (0.74 MBq)γ-32 P] ATP, 10 μM unlabeled ATP, and 2 μg substrate GST-IκBα (amino acids 1-54) and incubated at 30°C for 30 minutes. The reaction was terminated by boiling with SDS sample buffer for 5 minutes. Finally, the protein was resolved on 10% SDS-PAGE, the gel was dried, and the radioactive bands were visualized with a Storm 820. To determine the total amounts of IKK-α and IKK-β in each sample, 30 μg of whole-cell proteins was resolved on 10% SDS-PAGE, electrotransferred to a nitrocellulose membrane, and then blotted with either anti–IKK-α or anti–IKK-β antibodies.
NF-κB–dependent reporter gene transcription
To determine the effect of celastrol on TNF-, TNFR-, TRADD-, TRAF2-, NIK-, IKK-, and p65-induced NF-κB–dependent reporter gene transcription, we performed the secretory alkaline phosphatase (SEAP) assay as previously described.29 For the TAK1/TAB1 experiment, A293 cells (5 × 105 cells/well) were transiently transfected with the expression vectors for TAK1/TAB1 and pNF-κB-SEAP (0.5 μg) plasmids by the FuGENE 6 method. After 24 hours, cells were treated with indicated concentrations of celastrol, and conditioned medium was harvested after 6 hours for SEAP activity as described.
Results
The goal of this study was to investigate the effect of celastrol on the TNF signaling pathway that mediates apoptosis and inflammation. The structure of this triterpene is shown in Figure 1A. Human myeloid KBM-5 cells were used for most studies because these cells express both types of TNF receptors.
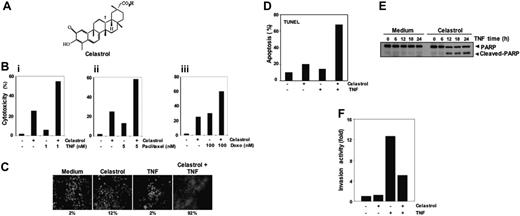
Celastrol and its effects. (A) Structure of triterpene, celastrol. (Bi-iii) Celastrol enhances TNF- and chemotherapeutic agent-induced cytotoxicity. In total, 10 000 cells were seeded in triplicate in 96-well plates. The cells were pretreated with 2.5 μM celastrol and then incubated with the indicated concentrations of TNF, paclitaxel, and doxorubicin for 24 hours. Cell viability was analyzed by the MTT method as described in “Materials and methods.” (C) Celastrol potentiates TNF-induced apoptosis. KBM-5 cells were pretreated with 2.5 μM celastrol for 6 hours and then incubated with 1 nM TNF for 16 hours. The cells were stained with a live/dead assay reagent for 30 minutes and then analyzed under a fluorescence microscope as described in “Materials and methods.” The results shown are representative of 3 independent experiments. (D) Cells were pretreated with 2.5 μM celastrol for 6 hours and then incubated with 1 nM TNF for 16 hours. Cells were fixed, stained with TUNEL assay reagent, and then analyzed with a flow cytometer for apoptotic effects. The results shown are representative of 2 independent experiments. (E) Cells were pretreated with 2.5 μM celastrol for 6 hours and then incubated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blotting using an anti-PARP antibody. The results shown are representative of 3 independent experiments. (F) Celastrol suppresses TNF-induced invasion activity. H1299 cells (2.5 × 104 cells) were seeded to the top chamber of a Matrigel invasion chamber system overnight in the absence of serum and then treated with celastrol. After incubation, the cells were treated with TNF in the presence of 1% serum and then assayed for invasion as described in “Materials and methods.” Results are expressed as fold activity of the untreated control.
Celastrol and its effects. (A) Structure of triterpene, celastrol. (Bi-iii) Celastrol enhances TNF- and chemotherapeutic agent-induced cytotoxicity. In total, 10 000 cells were seeded in triplicate in 96-well plates. The cells were pretreated with 2.5 μM celastrol and then incubated with the indicated concentrations of TNF, paclitaxel, and doxorubicin for 24 hours. Cell viability was analyzed by the MTT method as described in “Materials and methods.” (C) Celastrol potentiates TNF-induced apoptosis. KBM-5 cells were pretreated with 2.5 μM celastrol for 6 hours and then incubated with 1 nM TNF for 16 hours. The cells were stained with a live/dead assay reagent for 30 minutes and then analyzed under a fluorescence microscope as described in “Materials and methods.” The results shown are representative of 3 independent experiments. (D) Cells were pretreated with 2.5 μM celastrol for 6 hours and then incubated with 1 nM TNF for 16 hours. Cells were fixed, stained with TUNEL assay reagent, and then analyzed with a flow cytometer for apoptotic effects. The results shown are representative of 2 independent experiments. (E) Cells were pretreated with 2.5 μM celastrol for 6 hours and then incubated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared and analyzed by Western blotting using an anti-PARP antibody. The results shown are representative of 3 independent experiments. (F) Celastrol suppresses TNF-induced invasion activity. H1299 cells (2.5 × 104 cells) were seeded to the top chamber of a Matrigel invasion chamber system overnight in the absence of serum and then treated with celastrol. After incubation, the cells were treated with TNF in the presence of 1% serum and then assayed for invasion as described in “Materials and methods.” Results are expressed as fold activity of the untreated control.
Celastrol potentiates the apoptotic effects of TNF
NF-κB activation inhibits apoptosis induced by TNF and chemotherapeutic agents.30 Therefore, we investigated whether celastrol would modulate the apoptosis induced by TNF and chemotherapeutic agents in KBM-5 cells. The effect of celastrol on TNF- and chemotherapeutic agent-induced apoptosis was examined by the MTT assay. We found that celastrol enhanced the cytotoxic effects of TNF, paclitaxel, and doxorubicin (Figure 1Bi-iii). The live/dead assay, which measures intracellular esterase activity and plasma membrane integrity, also indicated that celastrol up-regulates TNF-induced apoptosis from 2% to 92% (Figure 1C). Results of TUNEL staining confirmed that TNF-induced apoptosis was enhanced by incubation with celastrol (Figure 1D). By using PARP cleavage assay, we further found that the enhanced cytotoxicity was due to apoptosis (Figure 1E). These results together indicate that celastrol potentiates the apoptotic effect of TNF.
Celastrol suppresses TNF-induced tumor-cell invasion activity
It is known that TNF can induce tumor metastasis-related genes, such as MMP9, COX2, and ICAM1.31 To investigate the effect of celastrol on TNF-induced metastatic activity, we examined the invasive activity in vitro. For this study, we seeded the tumor cells into the upper wells of a Matrigel invasion chamber in the absence of serum. The cells were pretreated with celastrol and then treated with TNF in the presence of 1% serum and celastrol. As shown in Figure 1F, TNF-induced tumor-cell invasion by almost 12-fold, and celastrol suppressed this activity.
Celastrol inhibits TNF-induced expression of antiapoptotic gene products
Because apoptosis is regulated by antiapoptotic proteins, such as IAP1, IAP2, Bcl-2, Bcl-XL, c-FLIP, and survivin,32 we examined whether celastrol can modulate the expression of these antiapoptotic gene products induced by TNF. The results of Western blot analysis showed that TNF induced the expression of these antiapoptotic proteins in a time-dependent manner and that celastrol suppressed the expression (Figure 2A).
Celastrol inhibits TNF-induced NF-κB-regulated gene products. (A) Celastrol inhibits the expression of antiapoptotic gene products such as survivin, IAP1, IAP2, Bcl-XL, Bcl-2, and c-FLIP. KBM-5 cells (2 × 106/mL) were left untreated or were incubated with 2.5 μM celastrol for 6 hours and then treated with 1 nM TNF for different amounts of time. Whole-cell extracts were prepared, and 30 μg of the whole-cell lysate was analyzed by Western blotting using antibodies against IAP1, IAP2, Bcl-xl, Bcl-2, cFLIP, and survivin as indicated. (B) Celastrol inhibits COX-2 and cyclin D1 expression induced by TNF. KBM-5 cells (2 × 106/mL) were left untreated or were incubated with 2.5 μM celastrol for 6 hours and then treated with 1 nM TNF for different times. Whole-cell extracts were prepared, and 30 μg of the whole-cell lysate was analyzed by Western blot analysis using antibodies against COX-2 and cyclin D1. (C) Celastrol inhibits VEGF and MMP-9 expression induced by TNF. KBM-5 cells (2 × 106/mL) were left untreated or were incubated with 2.5 μM celastrol for 6 hours and then treated with 1 nM TNF for different times. Whole-cell extracts were prepared, and 30 μg of the whole-cell lysate was analyzed by Western blot analysis using antibodies against MMP-9 and VEGF. Data are for a representative experiment of 3 independent experiments showing similar results.
Celastrol inhibits TNF-induced NF-κB-regulated gene products. (A) Celastrol inhibits the expression of antiapoptotic gene products such as survivin, IAP1, IAP2, Bcl-XL, Bcl-2, and c-FLIP. KBM-5 cells (2 × 106/mL) were left untreated or were incubated with 2.5 μM celastrol for 6 hours and then treated with 1 nM TNF for different amounts of time. Whole-cell extracts were prepared, and 30 μg of the whole-cell lysate was analyzed by Western blotting using antibodies against IAP1, IAP2, Bcl-xl, Bcl-2, cFLIP, and survivin as indicated. (B) Celastrol inhibits COX-2 and cyclin D1 expression induced by TNF. KBM-5 cells (2 × 106/mL) were left untreated or were incubated with 2.5 μM celastrol for 6 hours and then treated with 1 nM TNF for different times. Whole-cell extracts were prepared, and 30 μg of the whole-cell lysate was analyzed by Western blot analysis using antibodies against COX-2 and cyclin D1. (C) Celastrol inhibits VEGF and MMP-9 expression induced by TNF. KBM-5 cells (2 × 106/mL) were left untreated or were incubated with 2.5 μM celastrol for 6 hours and then treated with 1 nM TNF for different times. Whole-cell extracts were prepared, and 30 μg of the whole-cell lysate was analyzed by Western blot analysis using antibodies against MMP-9 and VEGF. Data are for a representative experiment of 3 independent experiments showing similar results.
Celastrol inhibits TNF-induced expression of gene products involved in proliferation
Both cyclin D1 and COX-2 are required for proliferation of different cell types.33,34 We investigated whether celastrol could modulate the expression of COX-2 and cyclin D1 gene products. We found that TNF treatment induced the expression of both proteins and that celastrol inhibited the expression (Figure 2B).
Celastrol inhibits TNF-induced expression of angiogenic and invasion-associated gene products
VEGF plays a critical role in angiogenesis,35 whereas MMP-9 has been shown to be required for cellular invasion.36 We investigated whether celastrol could modulate TNF-induced expression of VEGF and MMP-9 proteins. We found that TNF treatment did induce expression of VEGF and MMP-9 gene products in a time-dependent manner and that celastrol abolished the expression (Figure 2C).
Celastrol inhibits TNF-induced NF-κB activation
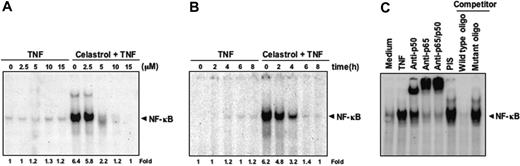
Our results until now show that celastrol potentiates TNF-induced apoptosis and down-regulates various gene products involved in cell proliferation, survival, angiogenesis, and invasion, all of which are known to be regulated by NF-κB activation.32 We examined whether celastrol affects TNF-induced NF-κB activation. Cells were pretreated with different doses of celastrol for 6 hours and then stimulated with 0.1 nM TNF for 30 minutes. As indicated by EMSA, celastrol suppressed TNF-induced NF-κB activation in a dose-dependent manner (Figure 3A). Celastrol by itself did not activate NF-κB. Under these conditions, cells were fully viable as determined by the trypan blue dye exclusion test (data not shown).
Celastrol blocks NF-κB activation induced by TNF in a dose- and time-dependent manner. (A) KBM-5 cells were incubated with the indicated concentrations of celastrol for 6 hours and then treated with 0.1 nM TNF for 30 minutes. The nuclear extracts were assayed for NF-κB activation by EMSA. The results shown are representative of 3 independent experiments. (B) KBM-5 cells were preincubated with 5 μM celastrol for the indicated times and then treated with 0.1 nM TNF for 30 minutes. The nuclear extracts were prepared and assayed for NF-κB activation by EMSA. The results shown are representative of 3 independent experiments. (C) NF-κB induced by TNF is composed of p65 and p50 subunits. Nuclear extracts from untreated cells or cells treated with 0.1 nM TNF were incubated with the indicated antibodies, an unlabeled NF-κB oligoprobe, or a mutant oligoprobe. They were then assayed for NF-κB activation by EMSA.
Celastrol blocks NF-κB activation induced by TNF in a dose- and time-dependent manner. (A) KBM-5 cells were incubated with the indicated concentrations of celastrol for 6 hours and then treated with 0.1 nM TNF for 30 minutes. The nuclear extracts were assayed for NF-κB activation by EMSA. The results shown are representative of 3 independent experiments. (B) KBM-5 cells were preincubated with 5 μM celastrol for the indicated times and then treated with 0.1 nM TNF for 30 minutes. The nuclear extracts were prepared and assayed for NF-κB activation by EMSA. The results shown are representative of 3 independent experiments. (C) NF-κB induced by TNF is composed of p65 and p50 subunits. Nuclear extracts from untreated cells or cells treated with 0.1 nM TNF were incubated with the indicated antibodies, an unlabeled NF-κB oligoprobe, or a mutant oligoprobe. They were then assayed for NF-κB activation by EMSA.
We also investigated the length of incubation required for celastrol to suppress TNF-induced NF-κB activation. KBM-5 cells were incubated with 5 μM celastrol for up to 8 hours and then exposed to TNF. EMSA results showed celastrol by itself did not activate NF-κB and that TNF-induced NF-κB activation was inhibited by celastrol within 6 hours (Figure 3B). Because NF-κB is a complex of proteins, various combinations of Rel/NF-κB proteins constitute active NF-κB heterodimers that bind to a specific site in DNA sequences.37,38 To demonstrate that the band visualized by EMSA in TNF-treated cells was indeed NF-κB, we incubated nuclear extracts from TNF-stimulated cells with antibodies to either the p50 (NF-κB1) or p65 (RelA) subunit of NF-κB. Both shifted the major band to a higher molecular mass (Figure 3C), suggesting that the TNF-activated complex consisted of p50 and p65 subunits. PIS had no effect, and excess unlabeled NF-κB (100-fold) caused complete disappearance of the band. However, a mutant oligonucleotide of NF-κB did not affect NF-κB binding activity.
Inhibition of NF-κB activation by celastrol is not cell type specific
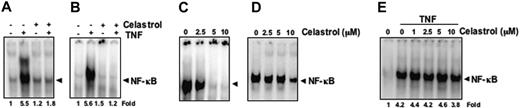
It has been reported that the NF-κB induction pathway in epithelial cells may differ from that in lymphoid cells.39 We therefore investigated whether celastrol inhibits NF-κB activation in different cell types. Celastrol completely inhibited TNF-induced NF-κB activation in lung adenocarcinoma (H1299) cells and embryonic kidney (A293) cells (Figure 4A-B), indicating a lack of cell-type specificity.
Celastrol suppresses TNF-induced NF-κB activation in different cell types. (A) Human lung carcinoma H1299 and (B) human embryonic kidney A293 cells were incubated with 5 μM celastrol for 6 hours, followed by incubation with 0.1 nM TNF for 30 minutes. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. The results shown are representative of 3 independent experiments. (C) Human multiple myeloma U266 and (D) bladder cancer 253JBV cells were incubated with the indicated concentrations of celastrol for 6 hours. Nuclear extracts were then prepared and analyzed for NF-κB activation by EMSA. (E) The direct effect of celastrol on NF-κB complex was investigated. Nuclear extracts were prepared from untreated cells or cells treated with 0.1 nM TNF and incubated for 30 minutes with the indicated concentrations of celastrol. They were then assayed for NF-κB activation by EMSA. The results shown are representative of 3 independent experiments.
Celastrol suppresses TNF-induced NF-κB activation in different cell types. (A) Human lung carcinoma H1299 and (B) human embryonic kidney A293 cells were incubated with 5 μM celastrol for 6 hours, followed by incubation with 0.1 nM TNF for 30 minutes. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA. The results shown are representative of 3 independent experiments. (C) Human multiple myeloma U266 and (D) bladder cancer 253JBV cells were incubated with the indicated concentrations of celastrol for 6 hours. Nuclear extracts were then prepared and analyzed for NF-κB activation by EMSA. (E) The direct effect of celastrol on NF-κB complex was investigated. Nuclear extracts were prepared from untreated cells or cells treated with 0.1 nM TNF and incubated for 30 minutes with the indicated concentrations of celastrol. They were then assayed for NF-κB activation by EMSA. The results shown are representative of 3 independent experiments.
Celastrol inhibits constitutive NF-κB activation
We then tested the effect of celastrol on NF-κB activation in human multiple myeloma (U266) and bladder cancer (253JBV) tumor cells, both of which express constitutively active NF-κB.40,41 Celastrol inhibited constitutively active NF-κB in both of these cells (Figure 4C-D). These results indicate a lack of cell-type specificity.
Celastrol does not directly affect binding of NF-κB to DNA
Some NF-κB inhibitors, including plumbagin, l-phenylalanine chloromethyl ketone (TPCK; a serine protease inhibitor), herbimycin A (a protein tyrosine kinase inhibitor), and caffeic acid phenethyl ester (CAPE), directly modify NF-κB to suppress its DNA binding.42-45 We examined whether celastrol mediates its effect through a similar mechanism. EMSA showed that celastrol did not modify the DNA-binding ability of NF-κB proteins prepared from TNF-treated cells (Figure 4E). These results suggest that celastrol inhibits NF-κB activation by a mechanism different from that of plumbagin, TPCK, herbimycin A, or CAPE.
Celastrol inhibits TNF-dependent IκBα degradation
Because IκBα degradation is required for NF-κB activation,20 we determined whether celastrol's inhibition of TNF-induced NF-κB activation was due to inhibition of IκBα degradation. We found that TNF induced IκBα degradation in control cells as early as 10 minutes, but in celastrol-pretreated cells, TNF had no effect on IκBα degradation (Figure 5A).
Inhibitory effects of celastrol. (A) Celastrol inhibits TNF-induced degradation of IκBα. KBM-5 cells were incubated with 5 μM celastrol for 6 hours and treated with 0.1 nM TNF for the indicated times. Cytoplasmic extracts were prepared and analyzed by Western blotting using antibodies against anti- IκBα. The results shown are representative of 2 or 3 independent experiments. Equal protein loading was evaluated by β-actin (bottom). (B) Celastrol blocks the phosphorylation of IκBα by TNF. Cells were preincubated with 5 μM celastrol for 6 hours, incubated with 50 μg/mL N-acetyl-leucyl-leucyl-norleucinal (ALLN) for 30 minutes, and then treated with 0.1 nM TNF for 10 minutes. Cytoplasmic extracts were fractionated and then subjected to Western blot analysis using phospho-specific anti-IκBα antibody. The same membrane was reblotted with β-actin antibody. (C) The effect of celastrol on the activation of IKK by TNF was investigated. KBM-5 cells were incubated with 5 μM celastrol for 6 hours, incubated with 50 μg/ml ALLN for 30 minutes, and then treated with 1 nM TNF for different time intervals. Whole-cell extracts were prepared, and extracts were immunoprecipitated with antibodies against IKK-α and IKK-β. Thereafter, the immune complex kinase assay was performed as described in “Materials and methods.” To examine the effect of celastrol on the level of expression of IKK proteins, whole-cell extracts were fractionated on SDS-PAGE and examined by Western blot analysis using anti–IKK-α and anti–IKK-β antibodies. The results shown are representative of 3 independent experiments. (D) Celastrol inhibits TNF-induced nuclear translocation and phosphorylation of p65. KBM-5 cells were either untreated or were pretreated with 5 μM of celastrol for 6 hours at 37°C and then treated with 0.1 nM TNF for the indicated times. Nuclear extracts were prepared and analyzed by Western blotting using antibodies against phospho-specific p65 and anti-p65. The results shown are representative of 2 or 3 independent experiments.
Inhibitory effects of celastrol. (A) Celastrol inhibits TNF-induced degradation of IκBα. KBM-5 cells were incubated with 5 μM celastrol for 6 hours and treated with 0.1 nM TNF for the indicated times. Cytoplasmic extracts were prepared and analyzed by Western blotting using antibodies against anti- IκBα. The results shown are representative of 2 or 3 independent experiments. Equal protein loading was evaluated by β-actin (bottom). (B) Celastrol blocks the phosphorylation of IκBα by TNF. Cells were preincubated with 5 μM celastrol for 6 hours, incubated with 50 μg/mL N-acetyl-leucyl-leucyl-norleucinal (ALLN) for 30 minutes, and then treated with 0.1 nM TNF for 10 minutes. Cytoplasmic extracts were fractionated and then subjected to Western blot analysis using phospho-specific anti-IκBα antibody. The same membrane was reblotted with β-actin antibody. (C) The effect of celastrol on the activation of IKK by TNF was investigated. KBM-5 cells were incubated with 5 μM celastrol for 6 hours, incubated with 50 μg/ml ALLN for 30 minutes, and then treated with 1 nM TNF for different time intervals. Whole-cell extracts were prepared, and extracts were immunoprecipitated with antibodies against IKK-α and IKK-β. Thereafter, the immune complex kinase assay was performed as described in “Materials and methods.” To examine the effect of celastrol on the level of expression of IKK proteins, whole-cell extracts were fractionated on SDS-PAGE and examined by Western blot analysis using anti–IKK-α and anti–IKK-β antibodies. The results shown are representative of 3 independent experiments. (D) Celastrol inhibits TNF-induced nuclear translocation and phosphorylation of p65. KBM-5 cells were either untreated or were pretreated with 5 μM of celastrol for 6 hours at 37°C and then treated with 0.1 nM TNF for the indicated times. Nuclear extracts were prepared and analyzed by Western blotting using antibodies against phospho-specific p65 and anti-p65. The results shown are representative of 2 or 3 independent experiments.
Celastrol inhibits TNF-dependent IκBα phosphorylation
To determine whether the inhibition of TNF-induced IκBα degradation was due to inhibition of IκBα phosphorylation, we used the proteasome inhibitor N-acetyl-leucyl-leucyl-norleucinal (ALLN) to block degradation of IκBα.46 Cells were pretreated with celastrol, treated with ALLN for 30 minutes, exposed to TNF for 10 minutes, and then examined for IκBα phosphorylation status by Western blot analysis using an antibody that recognizes the serine-phosphorylated form of IκBα. TNF alone slightly induced phosphorylation of IκBα, but when cells were pretreated with the inhibitor, the TNF-induced phosphorylation of IκBα was more pronounced (Figure 5B). Celastrol completely suppressed the IκBα phosphorylation induced by TNF in the presence of the proteasome inhibitor.
As with ALLN, celastrol has been shown to be a proteasome inhibitor.13 By inhibiting the degradation of IκBα, ALLN enhances the phosphorylation status of IκBα (Figure 5B). Unlike ALLN, however, celastrol did not enhance the TNF-induced phosphorylation status of IκBα (Figure 5B). These results indicate that celastrol must mediate its effects through a mechanism different from that of ALLN.
Celastrol inhibits TNF-induced IKK activation
Because celastrol inhibits the phosphorylation of IκBα, we tested its effect on TNF-induced IKK activation, which is required for TNF-induced phosphorylation of IκBα.46 Results from the immune complex kinase assay showed that TNF activated IKK as early as 2 minutes after treatment and that celastrol strongly suppressed this activation (Figure 5C). TNF and celastrol had no direct effect on the expression of either IKK-α or IKK-β proteins.
To evaluate whether celastrol suppressed IKK activity directly by binding the IKK protein or indirectly by suppressing the activation of IKK, we incubated whole-cell extracts from untreated and TNF-treated KBM-5 cells with up to 10 μM celastrol. Results from the immune complex kinase assay showed that celastrol did not directly affect the activity of IKK, suggesting that it modulates TNF-induced IKK activation (data not shown).
Celastrol inhibits TNF-induced nuclear translocation of p65
Degradation of IκBα is known to cause the nuclear translocation of the p65 subunit of NF-κB. We examined whether celastrol modulates TNF-induced nuclear translocation of p65. Western blot analysis showed that TNF induced nuclear translocation of p65 in a time-dependent manner in KBM-5 cells and that pretreatment with celastrol inhibited it (Figure 5D upper panel).
Celastrol inhibits TNF-induced phosphorylation of p65
TNF induces the phosphorylation of p65, which is required for its transcriptional activity.47 We examined whether celastrol modulates TNF-induced phosphorylation of p65. Western blot analysis showed that TNF induced the phosphorylation of p65 within 15 minutes and that celastrol strongly suppressed this phosphorylation (Figure 5D middle panel).
Celastrol represses TNF-induced NF-κB–dependent reporter gene expression
Although we determined by EMSA that celastrol inhibits NF-κB activation, DNA binding alone is not always associated with NF-κB–dependent gene transcription, suggesting that additional regulatory steps are involved.48,49 We examined whether celastrol modulates TNF-induced transcription of the NF-κB reporter activity. The results show that TNF induced the NF-κB reporter activity and that celastrol inhibited TNF-induced NF-κB reporter activity in a dose-dependent manner (Figure 6A).
Effects of celastrol on gene expression. (A) Celastrol inhibits TNF-induced NF-κB–dependent reporter gene (SEAP) expression. A293 cells were transiently transfected with an NF-κB–containing plasmid linked to the SEAP gene and then treated with the indicated concentrations of celastrol. After 24 hours in culture with 1 nM TNF, cell supernatants were collected and assayed for SEAP activity as described in “Materials and methods.” Results are expressed as fold activity over the activity of the vector control. (B) Celastrol inhibits NF-κB–dependent reporter gene expression induced by TNFR, TRADD, TRAF, NIK, IKKβ, and p65. A293 cells were transiently transfected with the indicated plasmids along with an NF-κB–containing plasmid linked to the SEAP gene and then left either untreated or treated with 2.5 μM celastrol for 6 hours. Cell supernatants were assayed for secreted alkaline phosphatase activity as described in “Materials and methods.” Results are expressed as fold activity over the activity of the vector control. Bars indicate standard deviation. (C) Celastrol suppresses TAK1/TAB1-induced NF-κB activation. A293 cells were transiently transfected with a TAK1/TAB1 expression plasmid along with NF-κB–containing plasmid. After 24 hours, cells were treated with indicated concentrations of celastrol and incubated with the relevant plasmid for an additional 6 hours. The supernatants of the culture medium were assayed for SEAP activity as described in “Materials and methods.” The results shown are representative of 3 independent experiments. (D) A schematic diagram of the effect of celastrol on TNF-induced NF-κB activation and apoptosis.
Effects of celastrol on gene expression. (A) Celastrol inhibits TNF-induced NF-κB–dependent reporter gene (SEAP) expression. A293 cells were transiently transfected with an NF-κB–containing plasmid linked to the SEAP gene and then treated with the indicated concentrations of celastrol. After 24 hours in culture with 1 nM TNF, cell supernatants were collected and assayed for SEAP activity as described in “Materials and methods.” Results are expressed as fold activity over the activity of the vector control. (B) Celastrol inhibits NF-κB–dependent reporter gene expression induced by TNFR, TRADD, TRAF, NIK, IKKβ, and p65. A293 cells were transiently transfected with the indicated plasmids along with an NF-κB–containing plasmid linked to the SEAP gene and then left either untreated or treated with 2.5 μM celastrol for 6 hours. Cell supernatants were assayed for secreted alkaline phosphatase activity as described in “Materials and methods.” Results are expressed as fold activity over the activity of the vector control. Bars indicate standard deviation. (C) Celastrol suppresses TAK1/TAB1-induced NF-κB activation. A293 cells were transiently transfected with a TAK1/TAB1 expression plasmid along with NF-κB–containing plasmid. After 24 hours, cells were treated with indicated concentrations of celastrol and incubated with the relevant plasmid for an additional 6 hours. The supernatants of the culture medium were assayed for SEAP activity as described in “Materials and methods.” The results shown are representative of 3 independent experiments. (D) A schematic diagram of the effect of celastrol on TNF-induced NF-κB activation and apoptosis.
We next investigated where celastrol acts in the sequence of TNFR1, TRADD, TRAF2, NIK, and IKK recruitment that characterizes TNF-induced NF-κB activation.50,51 In cells transfected with TNFR1, TRADD, TRAF2, NIK, IKKβ, and p65 plasmids, NF-κB-dependent SEAP expression was induced; celastrol substantially suppressed NF-κB–dependent SEAP expression in all cells except those transfected with p65 (Figure 6B). Because IKK activation can cause phosphorylation of IκBα and p65, we propose that celastrol inhibits NF-κB activation by inhibiting IKK activation.
Celastrol inhibits TAK1/TAB1-induced NF-κB–dependent reporter gene expression
Recent studies indicate that TAK1 plays a major role in TNF-induced NF-κB activation through its interaction with TAB1 and TAB2 and that it is needed for TNF-induced IKK activation.52 Therefore, we investigated whether celastrol suppresses TNF-induced NF-κB activation by inhibiting TAK1. As shown in Figure 6C, TAK1 activated NF-κB reporter activity, and celastrol inhibited the activation in a dose-dependent manner.
Discussion
This study was designed to investigate the effects of celastrol on TNF-induced cell signaling. We found that celastrol potentiated the apoptosis induced by TNF and chemotherapeutic agents and inhibited TNF-induced invasive activity. This effect correlated with down-regulation of various gene products that mediate cell proliferation, cell survival, invasion, and angiogenesis, all of which are known to be regulated by NF-κB. Celastrol suppressed not only inducible but also constitutive NF-κB activation. Suppression of NF-κB activation by celastrol is due to inhibition of TAK1 and IκBα kinase activation, which leads to inhibition of IκBα phosphorylation and degradation, suppression of p65 phosphorylation, and translocation to the nucleus; and inhibition of NF-κB–dependent reporter gene expression (Figure 6D).
We observed for the first time that celastrol potentiates the apoptotic effects of TNF, paclitaxel, and doxorubicin. We also investigated how celastrol causes this potentiation. The down-regulation of various antiapoptotic gene products by celastrol as shown here can sensitize the cells to the apoptotic effects of TNF. Nagase et al reported that celastrol can induce apoptosis in human leukemia HL-60 cells through the inhibition of topoisomerase II.11 We also found that celastrol down-modulated expression of cyclin D1 and COX-2. The previously reported antiproliferative effects of celastrol against a wide variety of tumor cells12,53 could be due to down-regulation of these gene products. Similarly, celastrol also suppressed gene products that have been implicated in angiogenesis and invasion. Thus, suppression of TNF-induced invasion of tumor cells could be due to down-regulation of MMP-9 as described here.
Our results show that celastrol suppresses TNF-induced NF-κB activation, as examined by DNA binding and by reporter assay. These results are consistent with a previous report that celastrol can suppress LPS-induced NF-κB–dependent reporter activity.12 Using immunocytochemistry, Zhang et al showed the suppression of TNF-induced NF-κB activation by celastrol.17 None of these investigators, however, examined the mechanism by which celastrol blocks NF-κB activation. We found that celastrol inhibits not only inducible NF-κB activation but also constitutively active NF-κB in tumor cells. Constitutive NF-κB activation has been found to be critical for the survival and proliferation of various tumor-cell types.20
We found that celastrol blocked the activation of NF-κB without directly interfering with the DNA-binding ability of NF-κB. Further analysis of the pathway indicated that celastrol inhibits TNF-induced activation of IKK. We also investigated how celastrol suppresses IKK activation. Several kinases, such as MEKK1,54 MEKK3,55 PKC,56 glycogen synthase kinase-3β,57 TAK1,52 PDK1,58 and Akt 59 have been reported to function upstream of IKK. Recent studies, however, indicate that TAK1 plays a major role in the canonical pathway activated by cytokines through its interaction with TAB1 and TAB2.52 TAK1 also has been shown to be recruited by the TNFR1 through TRADD, TRAF2, and RIP.60 Indeed, our study shows for the first time that TAK1-induced NF-κB activation is inhibited by celastrol, thereby suggesting that TAK1 is the main upstream stimulatory kinase modulated by celastrol.
We further observed that TNF-induced IκBα phosphorylation and degradation were suppressed by celastrol. Although celastrol has been shown to inhibit proteasome activity,13 we found that its ability to suppress TNF-induced IκBα degradation is mediated through inhibition of IκBα phosphorylation. Like ALLN, celastrol has been shown to be a proteasome inhibitor.13 By inhibiting the degradation of IκBα, ALLN enhances the phosphorylation status of IκBα. However, celastrol did not enhance the TNF-induced phosphorylation status of IκBα, indicating that celastrol must mediate its effects through mechanisms different from those of ALLN. Besides TNF-induced NF-κB reporter activity, the NF-κB activation induced TNFR1, TRADD, TRAF2, NIK, and IKK was also abrogated by celastrol. The NF-κB–dependent reporter activity induced by p65, however, was unaffected by celastrol, suggesting that this agent acts upstream to p65. Zhang et al recently found that this triterpene inhibits the expression of various adhesion molecules, such as selectin, vascular adhesion molecule (VCAM-1), and intercellular adhesion molecule (ICAM-1).17 The down-regulation of these adhesion molecules by celastrol is most likely due to the suppression of NF-κB activity as reported here. The down-regulation of inflammatory cytokines14,15 and iNOS16 also could be due to modulation of NF-κB activation.
Celastrol has also been shown to induce heat shock protein gene (hsp 70) expression via induction of heat shock transcription factor.19 Several heat shock proteins, including hsp 27, hsp 70, and hsp 90, have been found to inhibit NF-κB activation.61-63 Besides, many compounds that activate the heat shock response also inhibit NF-κB.64 Thus, it is possible that heat shock protein is involved in suppression of TNF-induced NF-κB activation by celastrol. Celastrol has been shown to have activity against several inflammatory diseases, including arthritis,5 Alzheimer disease,6 asthma,7 systemic lupus erythematosus,8,9 and rheumatoid arthritis.10 It also exhibits antitumor activity against a wide variety of tumor cells, including those from leukemia,11 gliomas,12 and prostate cancer.13 Our results indicate that the antitumor and anti-inflammatory effects of celastrol may be mediated through suppression of the TAK-1–regulated NF-κB pathway and NF-κB-regulated gene products.
Authorship
Contribution: G.S. performed experiments; K.S.A. performed experiments; M.K.P. performed experiments; and B.B.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
B.B.A. is a Ransom Horne Jr Professor of Cancer Research.
Correspondence: Bharat B. Aggarwal, Cytokine Research Laboratory, Department of Experimental Therapeutics, Unit 143, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: aggarwal@mdanderson.org.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), by the National Institutes of Health PO1 grant CA91844 on lung chemoprevention (B.B.A.), and by grant 5P30 CA016672-32 for flow cytometric analysis.
We would like to thank Walter Pagel for carefully proofreading the manuscript and providing valuable comments.