Abstract
In multiple myeloma (MM), the addition of thalidomide or bortezomib to the standard oral melphalan/prednisone combination significantly increased response rate and event-free survival. In this multicenter phase 1/2 trial, dosing, safety, and efficacy of the 4-drug combination, bortezomib, melphalan, prednisone, and thalidomide (VMPT) was determined. Bortezomib was administered at 3 dose levels (1.0 mg/m2, 1.3 mg/m2, or 1.6 mg/m2) on days 1, 4, 15, and 22; melphalan was given at a dose of 6 mg/m2 on days 1 through 5 and prednisone at 60 mg/m2 on days 1 through 5. Thalidomide was delivered at 50 mg on days 1 through 35. Each course was repeated every 35 days. The maximum tolerated dose of bortezomib was 1.3 mg/m2. Thirty patients with relapsed or refractory MM were enrolled; 20 patients (67%) achieved a partial response (PR) including 13 patients (43%) who achieved at least a very good PR. Among 14 patients who received VMPT as second-line treatment, the PR rate was 79% and the immunofixation-negative complete response rate 36%. The 1-year progression-free survival was 61%, and the 1-year survival from study entry was 84%. Grade 3 nonhematologic adverse events included infections (5 patients), fatigue (1), vasculitis (1), and peripheral neuropathy (2); no grade 4 toxicities were recorded. Initial results showed that VMPT is an effective salvage therapy with a very high proportion of responses. The incidence of neurotoxicities was unexpectedly low.
Introduction
Multiple myeloma (MM) is a malignant plasma cell proliferative disorder that accounts for more than 16 000 deaths each year in Europe.1 In the last 50 years, several combination chemotherapy regimens have been tested, but no regimen was better than the original melphalan and prednisone (MP) regimen.2 During the last 10 years, high-dose melphalan with hematopoietic stem cell support was the only option that significantly increased the rate of complete response (CR) and extended event-free and overall survival.3,4 Oral low-dose melphalan in elderly patients and high-dose melphalan in younger patients, therefore, became the standard of care. However, new drugs, such as thalidomide, lenalidomide, and bortezomib, are changing the therapeutic scenario of MM.5
Thalidomide, in combination with dexamethasone or chemotherapeutic agents, showed in vivo additive or synergistic activity, and often induced high rates of profound tumor regression, similar to those achieved after high-dose chemotherapy.6-10 The response rate of relapsed myeloma to thalidomide ranges from 25% to 35%.5,6 When thalidomide is used in combination with corticosteroids, the response rate increases to about 50% and to approximately 70% in combination with alkylating agents.5,7,8 In 2 independent randomized studies, the combination of MP plus thalidomide (MPT) not only increased the CR rate, but also significantly prolonged event-free and overall survival in comparison with the original MP.9,10 In the French study, MPT was also superior to a reduced intensity autologous transplantation procedure.9 These findings are now changing the treatment paradigm of elderly patients with myeloma.
Bortezomib is the first proteasome inhibitor to enter into clinical use. Preclinical trials have demonstrated in vitro synergy for bortezomib in combination with several cytotoxic agents, including melphalan.11-14 In newly diagnosed myeloma, the combination of bortezomib with MP was active and well tolerated in elderly patients (median age, 75 years), with a response rate of 89% including a remarkable 32% immunofixation-negative CR rate.15 Similarly, bortezomib plus thalidomide and dexamethasone significantly increased the response rate induced by thalidomide plus dexamethasone.16 Both bortezomib and thalidomide showed in vivo additive or synergistic activities when combined with melphalan, as did the combination of bortezomib with thalidomide.9-16 These observations provide the rationale for evaluating the tolerability and efficacy of the 4-drug combination bortezomib (Velcade; Janssen & Cilag, Milan, Italy), melphalan, prednisone, and thalidomide (VMPT). The primary goals of this study were to identify the most appropriate dose of bortezomib in combination with the MPT treatment regimen, which incorporates a reduced dose of thalidomide, and to determine the efficacy.
Patients, materials, and methods
Patient selection
Between November 2004 and August 2005, a total of 30 patients were enrolled in 7 Italian centers. All patients had measurable disease, defined as a monoclonal immunoglobulin concentration on serum electrophoresis of at least 10 g/L (1 g/dL) IgG or 5 g/L (0.5 g/dL) IgA, or urinary excretion of at least 200 mg monoclonal light chain per 24 hours. Patients had a relapse after a previous chemotherapy or the disease was refractory to salvage chemotherapy, as defined by progression during treatment or within 60 days after the completion of treatment.17
Inclusion criteria were as follows: patients had a relapse or were refractory after one or 2 lines of treatment; measurable disease; Karnofsky performance status 60% or higher; platelet count 75 × 109/L or higher; absolute neutrophil count at least 0.75 × 109/L; corrected serum calcium 3.5 mM (14 mg/dL) or less; serum hepatic aminotransferase levels less than or equal to 2.5 times the upper limit of normal; total bilirubin less than or equal to 1.5 times the upper limit; and calculated or measured creatinine clearance 0.33 mL/s (20 mL/min) or higher.
Exclusion criteria were: presence of another cancer; psychiatric disease; grade 2 peripheral neuropathy; or hypersensitivity to bortezomib, boron, mannitol, or thalidomide. Patients agreed to use effective contraception and women of childbearing age had a pregnancy test before enrollment. The study was approved by the institutional review board at each participating center. All patients gave written informed consent before entering the study, which was carried out in accordance with the Declaration of Helsinki.
Study design
This trial was a phase 1/2 multicenter, noncomparative, open-label study on the combination of bortezomib and melphalan/prednisone/thalidomide as salvage therapy in patients with advanced MM. The primary objectives were to determine an acceptable rate of toxicity by showing 30% or fewer patients with grade 4 neutropenia for at least 1 week, or a grade 4 hematologic toxicity except neutropenia, or any grade 3 nonhematologic toxicity; and the efficacy by showing at least 50% of patients in partial remission (PR) or at least 3% of patients in CR following the proposed regimen. The secondary objectives were to determine the durations of progression-free survival and survival.
Drug administration
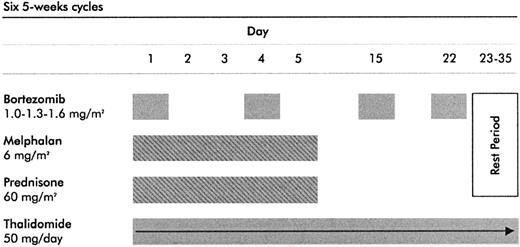
Bortezomib (Janssen & Cilag) was administered by intravenous bolus on days 1, 4, 15, and 22 at 3 dose levels: in the first 10 patients at 1.0 mg/m2, in the next 10 patients at 1.3 mg/m2, and in the last 10 patients at 1.6 mg/m2. Melphalan was given orally at the dose of 6 mg/m2/d for 5 days, prednisone was given orally at the dose of 60 mg/m2/d for 5 days, both followed by a 30-day rest period (days 6-35). Thalidomide (Pharmion, Windsor, United Kingdom) was administered orally at 50 mg/d for 35 days. Each cycle was repeated every 35 days, for a total of 6 courses (Figure 1).
Treatment schedule. The VMPT schedule is based on bortezomib at 3 dose levels (1.0 mg/m2, 1.3 mg/m2, or 1.6 mg/m2) on days 1, 4, 15, and 22; melphalan at 6 mg/m2 on days 1 through 5, prednisone at 60 mg/m2 on days 1 through 5; and thalidomide at 50 mg on days 1 through 35. Each course was repeated every 35 days.
Treatment schedule. The VMPT schedule is based on bortezomib at 3 dose levels (1.0 mg/m2, 1.3 mg/m2, or 1.6 mg/m2) on days 1, 4, 15, and 22; melphalan at 6 mg/m2 on days 1 through 5, prednisone at 60 mg/m2 on days 1 through 5; and thalidomide at 50 mg on days 1 through 35. Each course was repeated every 35 days.
Bortezomib dose was increased whenever at least 3 patients had completed 2 courses without a dose-limiting toxicity (DLT). DLT was defined as the occurrence of grade 4 neutropenia at least once a week,, or grade 4 hematologic toxicity except neutropenia, or any grade 3 or higher nonhematologic toxicity. The maximum tolerated dose (MTD) was defined as the highest dose level at which 30% or fewer patients experienced a DLT. Bortezomib dose reduction (from 1.6 to 1.3 to 1.0 and then to 0.7 mg/m2) and thalidomide dose reduction (from 50 mg/d to 50 mg every other day) were applied in patients experiencing grade 2 peripheral neuropathy or any grade 3 or higher nonhematologic or grade 4 hematologic toxicities.
Pretreatment, efficacy, and safety assessments
Pretreatment evaluations consisted of patient history and physical examination, electrocardiogram, and chest radiographs. Blood samples were collected at screening and before each intravenous treatment and again at the end of the study. A negative pregnancy test was required for all women of childbearing potential. A complete neurologic evaluation was done during the initial screening, during treatment as needed, and at the end of treatment. Assessments of both efficacy and safety were done every 5 weeks during chemotherapy regimens and every 2 months thereafter.
Treatment response was monitored by measurement of protein in serum and urine at each participating center every 5 weeks, using the uniformed response criteria of the International Myeloma Working Group to define responses.18 Briefly, a CR required disappearance of myeloma protein in serum and urine and negative immunofixation. A very good partial response (VGPR) required at least 90% reduction of myeloma protein in serum or urine myeloma protein level less than 100 mg/24 h. A PR required at least 50% reduction of myeloma protein in serum and a 90% decrease in urine. Stable disease (SD) was defined as all responses that did not meet criteria for CR, VGPR, PR, or progressive disease. Progressive disease was defined as an increase of 25% or greater in myeloma protein from baseline values. Responses were confirmed. Bone marrow plasmacytosis, skeletal disease, and serum calcium were included in the response evaluation. Progression-free survival was calculated from the time of enrollment until the date of progression, relapse, or death or the date the patient was last known to be in remission. Survival was calculated from the time of enrollment until the date of death or the date the patient was last known to be alive.
All adverse events were assessed at each visit and graded according to the National Cancer Institute Common Terminology Criteria (version 3).19 Causes of death were recorded as attributable to myeloma, study drugs, other causes, or a combination of these.
Statistical analysis
Time-to-event analysis was performed with the Kaplan-Meier method.20 Further subgroup analyses were done with the Cox model to estimate the hazard ratios (HRs) and the 95% confidence intervals (95% CIs) and to detect clinically relevant interactions between treatment and clinical factors.
The incidence of any adverse event was compared by the χ2 test or Fisher exact test when cell counts were lower than 5. The analyses were performed with SAS (version 8.2; SAS Institute, Cary, NC).
Results
Patients and dose escalation
At the time of the analysis, all 30 patients enrolled in the study had completed the assigned treatment schedule. Fourteen patients received VMPT as second-line therapy, and 16 patients received VMPT as third-line therapy. Twenty patients received a prior autologous transplant, 10 conventional chemotherapy, and 9 thalidomide-based regimens. Baseline demographics and other characteristics are listed in Table 1. The median number of cycles administered was 6 (range, 1-6).
Six of the 30 VMPT patients did not complete the assigned 6 cycles because of adverse events (2 patients had grade 3 neuropathy, 1 had grade 3 vasculitis, 1 had grade 2 confusion, 1 had grade 2 fatigue, and 1 had grade 3 pneumonia). Bortezomib was reduced from 1.0 to 0.7 mg/m2 for febrile neutropenia (1 patient); from 1.3 to 1.0 mg/m2 for herpes zoster reactivation (2 patients) and grade 4 thrombocytopenia (1 patient); from 1.6 to 1.3 or 1.0 mg/m2 for peripheral neuropathy (1 patient) and grade 4 thrombocytopenia (1 patient). Thalidomide was reduced from 50 mg/d to 50 mg every other day for grade 2 peripheral neuropathy in one patient and for grade 2 constipation in 2 patients.
DLTs during the dose escalation of bortezomib were as follows. In the first 10 patients who received bortezomib at 1.0 mg/m2, one patient experienced grade 3 pneumonia, one patient grade 3 febrile neutropenia, and one grade 3 vasculitis. In the next 10 patients who received bortezomib at 1.3 mg/m2, 3 patients experienced DLTs (2 grade 4 thrombocytopenia, 1 grade 4 anemia, and 2 grade 3 herpes zoster reactivation). In the last 10 patients who received bortezomib at 1.6 mg/m2, 4 patients experienced DLTs (2 grade 4 thrombocytopenia, 2 grade 3 peripheral neuropathy, 1 grade 3 esophagitis, and 1 grade 3 fatigue). Bortezomib at 1.3 mg/m2 was therefore considered the MTD.
Efficacy
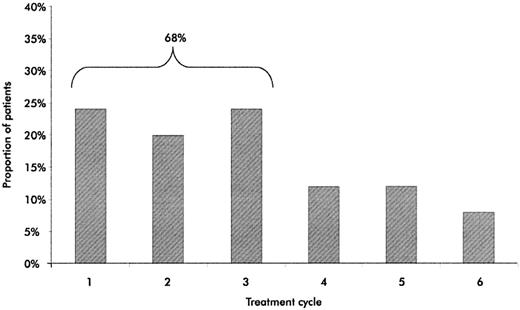
A high proportion of patients had CRs or VGPRs (Table 2). A CR or VGPR was achieved in 13 of 30 patients (43%) and at least a PR in 20 of 30 patients (67%). In the subgroup of patients who received second-line VMPT, immunofixation-negative CRs were observed in 5 of 14 subjects (36%) and CR or VGPR in 8 of 14 subjects (57%). The median time to the best response was 15 weeks (range, 35-210 days). The best response occurred within the first 3 cycles in 68% of patients (Figure 2). The response rate was not significantly different in the subgroups of patients treated with bortezomib at 1.0, 1.3, or 1.6 mg/m2.
Time to best response. The proportion of patients achieving their best response to VMPT during every cycle of therapy is shown.
Time to best response. The proportion of patients achieving their best response to VMPT during every cycle of therapy is shown.
The median duration of follow-up from study entry was 11 months (range, 8-17 ± 3.2 [SD]) for survivors. Progression, relapse, or death occurred in 15 of 30 patients (50%). The 1-year progression-free survival was 61% in all patients (Figure 3A). The 1-year progression-free survival among patients who received second-line VMPT was 100% as compared with 27% in patients receiving third-line VMPT (HR for second-line VMPT 0.17; 95% CI, 0.05-0.64; P = .009; Figure 3B). In patients who never received thalidomide before study entry, the HR of the progression-free survival was 0.29 (95% CI, 0.09-0.9; P = .03) in comparison with those who received prior thalidomide. The 1-year progression-free survival was 67.3% in patients who achieved at least a PR. The 1-year survival from the start of therapy among all patients was 84% (Figure 3C); 5 deaths were observed, all caused by disease progression. The 1-year survival from start of therapy was 100% in patients who achieved at least a PR.
Progression-free survival and overall survival. (A) Progression-free survival in the patients treated with VMPT. (B) Progression-free survival in the subgroup of patients who received VMPT as second- or third-line therapy. (C) Survival from study entry among all 30 patients.
Progression-free survival and overall survival. (A) Progression-free survival in the patients treated with VMPT. (B) Progression-free survival in the subgroup of patients who received VMPT as second- or third-line therapy. (C) Survival from study entry among all 30 patients.
Other secondary end points included additional measures of clinical benefit. None of the patients with at least a PR required transfusion after the first treatment cycle. Responses were also associated with increases in the platelet count, levels of normal immunoglobulins, and Karnofsky performance status scores.
Subgroup analyses did not show any statistical or clinical difference between responses and either age, β2-microglobulin, C-reactive protein, line of treatment, or dosage of bortezomib. Responses occurred in patients with or without abnormalities in chromosome 13 (71% versus 68%, P = .4). Serum albumin level less than 35 g/L (3.5 g/dL) was loosely associated with a lower response rate (25% versus 77%, P = .09).
Safety
The most common grade 1-2 adverse events were gastrointestinal symptoms, dermatologic rash, fatigue, sensory neuropathy, and pneumonia (Table 3). Gastrointestinal and dermatologic events were typically mild to moderate and were manageable with routine support. The most common grade 3 adverse events were neutropenia (23%), anemia (13%), thrombocytopenia (20%), herpes zoster reactivation (7%), pneumonia (3%), and neuropathy (7%). Grade 4 events included only hematologic toxicities, which were thrombocytopenia (13%) and neutropenia (20%). No patient had grade 4 neuropathy or nonhematologic toxicities. No death was reported as a consequence of adverse events. Eleven (37%) patients received granulocyte colony-stimulating factor (G-CSF) support. Among the 23 patients who did not have neuropathy before study entry, grade 1-2 neuropathy developed in 8 patients and grade 3 neuropathy developed in one patient. Among 7 patients with pre-existing grade 1 neuropathy, neuropathy worsened to grade 2 in 2 patients, to grade 3 in one patient, and resolved in one patient. All grade 3 neuropathies were observed in the subgroup of patients who received bortezomib at 1.6 mg/m2. After the introduction of acyclovir prophylaxis, no herpes zoster reactivation was recorded. The majority of grade 3-4 adverse events occurred during the first 2 cycles of therapy (36 events during cycles 1-2 versus 11 events during cycles 3-6, P = .04) and in patients who received third-line VMPT of therapy (27 events) compared with second-line VMPT therapy (10 events; P = .06).
Discussion
In this phase 1/2 trial, we evaluated the efficacy and safety profile of the 4-drug combination, VMPT, in patients with relapsed, refractory MM. The CR or VGPR rate was 43%. In the subgroup of patients who received VMPT as second-line therapy, the immunofixation-negative CR rate was 36%. The median progression-free survival was 12 months.
Because CR or VGPR are considered important surrogates for remission duration and survival,3,4,21,22 the high proportion of such responses observed after VMPT were encouraging. The combination of bortezomib plus melphalan in advanced myeloma induced a CR or VGPR rate of 12%,23,24 whereas the combination of bortezomib with thalidomide and dexamethasone induced a near-CR rate of 16%.25 The VMPT regimen almost doubled these numbers. Interestingly, the response rate induced by VMPT in advanced myeloma was quite similar to the response rate achieved by MPT in patients with newly diagnosed myeloma. Following MPT, the PR rate was 76% and the VGPR rate was 36%.10
There was a peculiar pattern of response to VMPT. Responses occurred rapidly, with an improvement in the quality of responses (VGPR or CR) observed with subsequent treatment cycles. A maximum response was achieved in 32% of patients after the third cycle. In the international phase 3 study of bortezomib (assessment of proteasome inhibition for extending remissions [APEX]) or in the phase 1/2 study of bortezomib, melphalan, and prednisone (VMP), best responses continued to improve over the treatment course, with approximately 30% of patients achieving maximum M-protein reduction beyond the first 18 weeks of therapy.15,26 These data suggest that a prolongation of VMPT from 6 to 9 treatment cycles might further improve results.
Responsiveness to bortezomib did not correlate with most of the standard prognostic factors, including chromosome 13 deletion, which predicts a poor outcome with conventional therapy.27,28 It is possible that the unique mechanism of action of bortezomib overcomes the influence of these adverse prognostic factors. This is consistent with the results of both the APEX and the VMP trials, in which bortezomib appeared to overcome the adverse impact of cytogenetic abnormalities on response rate.15,29
Whether a sequential single-agent treatment would yield similar survival benefit with less toxicity in comparison with a more complex combinational regimen administered at diagnosis, remains an important unanswered question. Fermand et al recently analyzed the activity of high-dose chemotherapy in comparison with conventional treatment. No survival influence of treatment choice was reported, but the period of time without symptoms, treatment, and treatment toxicity was significantly longer in patients who received the more complex high-dose regimen.30 Accordingly, if a combinational approach is superior to single-agent therapy, it might be considered at diagnosis, when there is the best chance to induce a prolonged remission duration. A sequential approach might then be considered at first and subsequent relapses, when less intense and more palliative approaches might be suggested.
Most adverse events could be managed with the use of standard approaches. No nonhematologic grade 4 adverse event was shown. Severe myelosuppression was observed in 50% of patients, with febrile neutropenia occurring in less than 5%. Neutropenia was the most common adverse event; thrombocytopenia was less common. In the APEX and VMP trials, bortezomib was administered at days 1, 4, 8, and 11, every 21 days, and thrombocytopenia was the most common adverse event.15,31 In the VMPT trial, bortezomib was delivered at days 1, 4, 15, and 22, every 35 days, and thrombocytopenia was less evident.
No cases of grade 4 peripheral neuropathy occurred. Severe grade 3 peripheral sensory neuropathy was observed in 2 patients only, with both patients found to be in the subgroup of patients who received bortezomib at 1.6 mg/m2. In the APEX and VMP trials, the incidence of grade 3 neuropathy was 12% and 17%, respectively.15,31 In the VMPT trial, the incidence was 6% despite the concomitant administration of thalidomide. In a previous phase 1/2 dose-escalation study of bortezomib, thalidomide, and dexamethasone (VTD) in patients with advanced myeloma, no MTD of thalidomide was reached in combination with bortezomib at 1.0 mg/m2, whereas the MTD of thalidomide was 150 mg/d in combination with bortezomib at 1.3 mg/m2.25 In the VMPT study, a reduced dose intensity of both bortezomib (1.3 mg/m2 at days 1, 4, 15, 22) and thalidomide (50 mg/d) seems safe and very well tolerated.
Despite the absence of any anticoagulant prophylaxis, no deep vein thrombosis was reported. These data are consistent with previous studies, which showed that relapsed and refractory patients appear to have a lower risk of deep vein thrombosis in comparison with patients with newly diagnosed disease.32
In conclusion, the novel proteasome inhibitor bortezomib in combination with melphalan, prednisone, and thalidomide induced clinically significant responses, with manageable toxic effects, in patients with relapsed or relapsed and refractory myeloma. A national, randomized, multicenter phase 3 trial is currently evaluating this combination in patients with disease at an earlier stage.
Authorship
Contribution: A.P. and M.B. designed the study, supervised its conduct and data analysis, and wrote the report; P.M. reviewed and commented on the draft of the report; M.T.A., S.B., P.F., I.A., and F.C. recruited patients, analyzed data, and assisted in writing the manuscript; and G.B., P.P., N.P., V.C., C.C., T.C., and F.M. recruited patients.
Conflict-of-interest disclosure: A.P. and M.B. have received scientific adviser board and lecture fees from Pharmion and Janssen-Cilag. The other authors declare that they have no conflict of interest.
A list of the participating members of GIMEMA appears as a data supplement to the online version of this article (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Antonio Palumbo, Divisione di Ematologia dell'Università di Torino, Azienda Ospedaliera S. Giovanni Battista, Via Genova 3, 10126 Torino, Italy; e-mail: appalumbo@yahoo.com.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was sponsored by the Università degli Studi di Torino, Turin, Italy, and supported in part by research funding from Janssen-Cilag (M.B.) and by Fondazione Neoplasie Sangue Onlus, Associazione Italiana Leucemie, Compagnia di S Paolo, Fondazione Cassa di Risparmio di Torino, Ministero dell'Università e della Ricerca (MIUR), and Consiglio Nazionale delle Ricerche (CNR).
We thank the patients, nurses, physicians, the Clinical Trial Office staff (Tiziana Marangon, Federica Leotta, Antonella Bono, Maria Josè Fornaro), and Rosalba Rosato from CPO Piemonte.