Abstract
Transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) is a member of the tumor necrosis factor (TNF) receptor family that serves as a receptor for B-cell activating factor of the TNF family (BAFF) and as a proliferation-inducing ligand (APRIL). Although TACI is reported to function as a positive or negative regulator for B-cell responses, its roles remain elusive. Experiments using TACI siRNA into B cells indicated that TACI positively regulated APRIL-induced IgA production in collaboration with heparan sulfate proteoglycans (HSPG). Furthermore, TACI negatively regulated BAFF-induced B-cell proliferation and production of IgA and IgG. In addition, B cells treated with heparitinase to denature HSPG showed that HSPG is essential for APRIL-induced B-cell responses such as B-cell proliferation, IgG and IgA production, induction of activation-induced cytidine deaminase (AID), and noncanonical NF-κB2. In contrast, phosphorylation of physiological AID kinase, protein kinase A (PKA), was dependent on TACI. Importantly, coligation of TACI and HSPG by specific antibodies, but not by TACI or HSPG ligation itself, could induce the phosphorylation of PKA and IgA production instead of APRIL. Our findings indicate that simultaneous binding of TACI and HSPG on B cells with APRIL is crucial for IgA production.
Introduction
Tumor necrosis factor (TNF) family ligands, a proliferation-inducing ligand (APRIL, CD256), and B-cell activating factor of TNF family (BAFF, also known as BLyS, TALL-1, THANK, zTNF4, TNFSF13b, and CD257) are implicated in several immunologic phenomena such as peripheral B-cell survival, CD154 (CD40L)–independent antibody isotype switching and production, autoimmunity, and tumor cell growth.1,2 BAFF and APRIL bind to 2 receptors, BCMA (B-cell maturation antigen [TNFRSF 17 and CD269]) and TACI (transmembrane activator and calcium-modulator and cyclophilin ligand interactor [TNFRSF 13B and CD267]).3-5 BAFF binds selectively to the third BAFF receptor, BAFF-R (TNFRSF 13C and CD268).6,7 All these receptors are TNF receptor family molecules. On the other hand, APRIL interacts with heparan sulfate proteoglycans (HSPGs), which are structurally unrelated to TNF receptors and are likely a third receptor for APRIL.8,9
Studies on transgenic and knockout mice have indicated that BAFF/BAFF-R interactions are primarily responsible for B-cell survival and responses because the BAFF-deficient phenotype is characterized by a reduced number of splenic B cells and by insensitivity to T cell–dependent and T cell–independent antibody production10 similar to that in BAFF-R–deficient mice.11,12 In contrast, TACI-deficient mice show mature B-cell hyperplasia and autoimmunity, and TACI can directly induce apoptosis under certain conditions,13-15 suggesting that TACI is a negative regulator of BAFF signaling in B-cell survival and responses. However, it is also reported that TACI-deficient mice exhibit reduced immunoglobulin A (IgA) production and compromised humoral responses to T-independent antigens (TI-Ags)13 and that defects in TACI are associated with 2 forms of human immunodeficiency, common variable immunodeficiency (CVID) and IgA deficiency.16,17 Thus, the real roles of TACI in B-cell responses remain obscure. On the other hand, studies on APRIL transgenic mice showed enhanced humoral responses to T-dependent (TD) and TI-Ags and a gradual increase in serum IgA level.2,18 APRIL knockout mice have low serum IgA levels and impaired IgA responses, though conflicting results are reported.2,19 In addition, in vitro studies demonstrated that APRIL enhances B-cell proliferation, plasmablast survival,20 and class switch recombination (CSR) to IgG and IgA through the up-regulation of activation-induced cytidine deaminase (AID).21
Given the potential importance of TACI and APRIL in IgA production, we performed a series of experiments using a small interference RNA (siRNA) technique to knock down TACI and heparitinase treatment to denature HSPG on human peripheral blood B cells to assess the functional aspects of TACI and HSPG in B-cell responses. The studies reported here show the mutually close relationship between TACI and HSPG in APRIL-induced B-cell responses, especially in IgA production.
Materials and methods
Antibodies and reagents
The following antibodies were used: NF-κB1/p65, NF-κB2/p52, Lamin A, and AID (Santa Cruz Biotechnology, Santa Cruz, CA); polyclonal human TACI (Active Motif, Carlsbad, CA), PKA C-α, and phospho-PKA C (Thr197) (Cell Signaling Technology, Beverly, MA); β-actin and FLAG-M2 (Sigma-Aldrich, St Louis, MO); HSPG (10E4, mouse IgM, κ; Seikagaku, Tokyo, Japan); human BCMA (Alexis, Läufelfingen, Switzerland); human CD40 and CD19 (eBioscience, San Diego, CA); human IgA (Dako, Copenhagen, Denmark); human Igκ and Igλ, and mouse Igκ (BD PharMingen, San Diego, CA); and control mouse IgM and IgG2a (Sigma-Aldrich). The following reagents were used: heparitinase (MP Bioscience, Solon, OH); human IL-4, human soluble CD40L, human BAFF, and TGF-β (PeproTech, Rocky Hill, NJ); human APRIL-FLAG fusion protein (Mega-APRIL; Alexis); and 8-bromo-cAMP (Calbiochem, La Jolla, CA). Human BAFF-FLAG fusion protein was prepared as described previously.22 Anti–human TACI mAb (11H3, mouse IgG2a,κ) was prepared by immunization with human TACI full-length cDNA-transfected cells. The specificity and agonistic activity of anti–human TACI mAb are demonstrated in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Cell preparation and cell cultures
Human peripheral blood mononuclear cells were isolated from buffy coats by using centrifugation with Ficoll-Hypaque, and purified B cells were isolated by depletion of non–B-cell populations using a B-cell isolation kit and auto-MACS (Miltenyi Biotec, Bergisch Gladbach, Germany). The resultant B-cell population was less than 2% CD14+, less than 1% CD3+, less than 2% CD57+, less than 2% IgA+, and greater than 95% CD20+. IgG-negative B cells were prepared by using biotinylated anti–human IgG (BD PharMingen) and auto-MACS. The resultant IgG-negative B-cell population was less than 3% IgG+. B cells were cultured in RPMI 1640 medium supplemented with 25 mM N-2-hydroxyetylpiperazine-N′-ethanesulfonic acid (HEPES), 10% fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 5.5 × 10−2 mM β-mercaptoethanol, 100 U/mL penicillin, and 100 U/mL streptomycin (all from Invitrogen, Carlsbad, CA). To stimulate B cells with APRIL and BAFF, the dose of APRIL and BAFF (1, 2, 4, or 8 μg/mL) was first titrated, and it was determined that the doses at 8 μg/mL for APRIL and 4 μg/mL for BAFF were most effective to induce cell proliferation and IgA and IgG production of human peripheral blood B cells in the present system. In some experiments, B cells were treated with heparitinase (10 U/mL) for 10 minutes at 37°C.
Gene transfer of siRNA
TACI siRNA (214802, 5 μg; Ambion, Austin, TX) or control siRNA (4611, 5 μg; Ambion) was transfected into B cells (4 × 106 cells) with the use of the human B-cell nucleofector kit and nucleofector (Amaxa Biosystems, Gaithersburg, MD) and the U-15 program. After 16-hour incubation, cells were subjected to experiments for evaluation of TACI knockdown.
Flow cytometric analysis
After incubation of arbitrary antibody with 2 μg/106 cells for 20 minutes on ice, the cells were washed and resuspended in propidium iodide solution and analyzed using FACSCalibur (Becton Dickinson, Mountain View, CA) and associated CellQuest (Becton Dickinson) software. In some experiments, after incubation of 10 ng APRIL-FLAG or BAFF-FLAG with 1 × 106 cells for 30 minutes at 37°C, the cells were washed and incubated with anti–FLAG M2 mAb for 20 minutes on ice. Fluorescein isothiocyanate (FITC)–labeled goat antibody to mouse IgG (Caltag, Burlingame, CA) was used as a secondary antibody. Mouse IgG control antibody was used to evaluate the background.
Proliferation assay
Sixteen hours after transfection with TACI siRNA or control siRNA, B cells were cultured in a 96-well plate (1 × 105/well) with anti-BCR antibodies (anti-Igκ and anti-Igλ, 0.5 μg/mL each), CD40L (2 μg/mL), BAFF (4 μg/mL), APRIL (8 μg/mL), anti-TACI mAb (5 μg/mL), or control mouse IgG2a (5 μg/mL) in the presence or absence of IL-4 (20 U/mL) and TGF-β (1 ng/mL). B-cell proliferation was quantitated by pulsing the cells during the last 18 hours of 72-hour culture with 0.5 μCi (18.5 kBq) per well of [3H] thymidine and using a liquid scintillation beta counter (TopCount NXT; Perkin Elmer, Wellesley, MA).
Detection and quantification of in vitro immunoglobulin secretion
After culture of IgG-negative or IgA-negative B cells in a 96-well plate (1 × 105/well) for 10 days with arbitrary stimulations, the amount of IgG, IgA, or IgM secreted in the culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA) using anti–human Ig (Southern Biotechnology, Birmingham, AL) as the capture antibody, and horseradish peroxidase (HRP)–labeled goat anti–human IgG (ICN Biomedicals) HRP-labeled goat anti–human IgA or HRP-labeled goat anti–human IgM (Sigma-Aldrich) as the detector antibody. After the addition of p-nitrophenyl phosphate substrate (Sigma-Aldrich), the amount of IgG, IgA, or IgM was measured by spectrophotometry at 490 nm using a microplate reader (model 550; Bio-Rad, Hercules, CA).
Immunoblot analyses
To prepare whole cell lysates, cells were washed with phosphate-buffered saline (PBS), suspended in 0.5% sodium dodecyl sulfate (SDS) solution, and boiled for 5 minutes. To prepare nuclear extracts, cells were treated with Nuclear Extract Kit (Active Motif). Proteins (5-8 μg) were separated electrophoretically by SDS-PAGE and then were transferred onto an Immobilon-P (Millipore, Bedford, MA) membrane. Immunoblots were probed using arbitrary antibody and developed with HRP-labeled secondary antibody (Amersham Biosciences, Piscataway, NJ). Blotting was visualized and subjected to densitometric analysis with the use of a LumiVision analyzer (Taitec, Saitama, Japan).
Statistical analysis
All data were expressed as mean ± SD. Differences between groups were examined for statistical significance using the paired t test. P < .05 denoted a statistically significant difference.
Results
TACI and HSPG contribute equally to the binding of APRIL to B cells
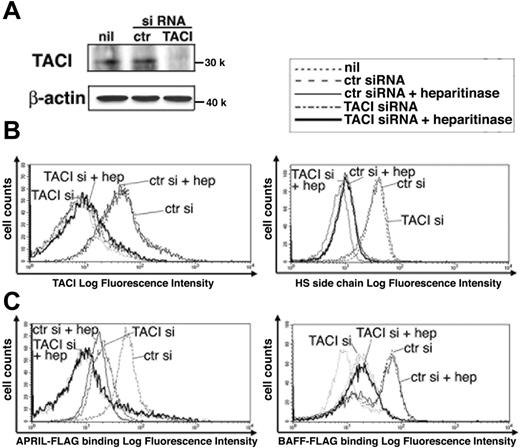
To clarify the roles of TACI and HSPG in APRIL-induced B-cell responses, we first examined the dependence on TACI and HSPG in APRIL-binding to human peripheral blood B cells by using an siRNA approach to knock down TACI or treatment with heparitinase to cut the heparan sulfate side chain of HSPG, which is crucial for APRIL binding.8 An exogenously supplied siRNA for TACI resulted in complete loss of TACI expression at the protein level (Figure 1A) and its cell surface expression (Figure 1B, left panel) compared with control siRNA, whereas the binding of monoclonal antibody (mAb) specific for the heparan sulfate side chain of HSPG was unaffected (Figure 1B, right panel). Similarly, heparitinase-treated B cells showed almost no binding of anti–heparan sulfate mAb (Figure 1B, right panel) but did show unaffected binding of anti-TACI mAb (Figure 1B, left panel). TACI surface expression on B cells was unchanged by treatment with heparitinase for 24, 48, and 72 hours (data not shown). Although BCMA shares the role of APRIL receptor, few or no BCMA-positive cells constituted human peripheral blood B cells, and BAFF-R and CD40 expression patterns did not change by heparitinase or TACI siRNA treatment (Figure S2). Under these conditions, we compared the binding ability of BAFF and APRIL to B cells by using FLAG-tagged recombinant proteins. The proportion of APRIL bound to B cells was equally reduced by the depletion of TACI or the denaturation of HSPG, each of which showed complete loss of its binding (Figure 1C, left panel). On the other hand, TACI knockdown led to reduced binding of BAFF but to no change after denaturation of HSPG (Figure 1C, right panel). These results suggest that HSPG and TACI contribute equally to APRIL binding to B cells.
Effects of TACI siRNA and heparitinase on APRIL and BAFF binding to B cells. (A) Down-regulation of TACI by siRNA. Control or TACI siRNA was transfected into human peripheral blood B cells, as described in “Materials and methods,” and was subjected to immunoblot analysis probed by polyclonal anti-TACI antibody. β-Actin represents the loading control. (B) Cell surface expression of TACI and HSPG. B cells transfected with TACI siRNA or control siRNA were stained with anti-TACI mAb (11H3; left) or anti–heparan sulfate side chain of HSPG mAb (10E4; right) in the presence or absence of heparitinase (10 U/mL) for 10 minutes at 37°C. (C) Reduced binding ability of APRIL and BAFF by the defect of TACI and HSPG. Cells were treated as in panel B and were stained with FLAG-tagged APRIL (left) or BAFF (right). Stained cells were analyzed by flow cytometry. Ctr indicates control siRNA; hep, heparitinase treatment. Data are representative of 3 independent experiments with similar results.
Effects of TACI siRNA and heparitinase on APRIL and BAFF binding to B cells. (A) Down-regulation of TACI by siRNA. Control or TACI siRNA was transfected into human peripheral blood B cells, as described in “Materials and methods,” and was subjected to immunoblot analysis probed by polyclonal anti-TACI antibody. β-Actin represents the loading control. (B) Cell surface expression of TACI and HSPG. B cells transfected with TACI siRNA or control siRNA were stained with anti-TACI mAb (11H3; left) or anti–heparan sulfate side chain of HSPG mAb (10E4; right) in the presence or absence of heparitinase (10 U/mL) for 10 minutes at 37°C. (C) Reduced binding ability of APRIL and BAFF by the defect of TACI and HSPG. Cells were treated as in panel B and were stained with FLAG-tagged APRIL (left) or BAFF (right). Stained cells were analyzed by flow cytometry. Ctr indicates control siRNA; hep, heparitinase treatment. Data are representative of 3 independent experiments with similar results.
TACI and HSPG are required for APRIL-induced IgA production, whereas TACI inhibits BAFF-induced B-cell proliferation and production of IgA and IgG
To determine the contribution of TACI and HSPG in APRIL-induced B-cell responses such as cell growth and immunoglobulin CSR, we evaluated cell proliferation, IgA secretion from IgA-negative B cells, and IgG secretion from IgG-negative B cells after treatment with TACI siRNA and heparitinase. B cells with control siRNA showed enhanced B-cell proliferation and secretion of IgA and IgG after stimulation with CD40L, BAFF, and APRIL in the presence of IL-4, TGF-β, and anti-BCR antibodies (Figure 2A, open bars). BAFF-induced B-cell proliferation and secretion of IgA and IgG were significantly enhanced to almost the same level as CD40L by TACI siRNA but not by heparitinase treatment (Figure 2A, lane 5). These results clearly indicate that TACI negatively regulates BAFF-induced B-cell responses. On the other hand, APRIL-induced B-cell responses were almost completely inhibited by heparitinase treatment (Figure 2A, lane 6). Importantly, TACI knockdown did not result in changes in B-cell proliferation, almost completely inhibited IgA secretion, and slightly suppressed IgG secretion by APRIL (Figure 2A, lane 6). To confirm that APRIL-induced IgA and IgG secretion observed here reflects APRIL-induced IgA and IgG CSR, respectively, we counted the number of viable cells and IgM secretion after culture (Table S1), calculated per cell immunoglobulin secretion based on Figure 2A and Table S1, and normalized the levels of secreted IgA and IgG to levels of IgM secretion (Figure 2B). APRIL increased the IgA/IgM ratio, which was reduced by treatment with TACI siRNA and heparitinase, though APRIL also increased the IgG/IgM ratio, which was reduced by treatment with heparitinase but not by TACI siRNA (Figure 2B, lane 6). In agreement with the data that APRIL-induced IgA CSR and production was abolished by TACI siRNA, flow cytometric analysis showed that 9.8% of untreated B cells were positive for IgA after 48-hour stimulation with APRIL to a level comparable to that of BAFF stimulation (Figure 2C). In contrast, only 2.3% or 2.8% of B cells were positive for IgA after treatment with heparitinase or TACI siRNA, respectively (Figure 2C). It should be noted that agonistic anti-TACI mAb itself failed to elicit B-cell responses (Figure 2A, lane 7). Thus, these results suggest that HSPG is essential for APRIL-induced B-cell responses and that TACI positively regulates APRIL-induced IgA CSR and production but not B-cell proliferation or IgG production. APRIL binding to TACI and HSPG is indispensable for IgA production.
Effects of TACI siRNA and heparitinase on APRIL- and BAFF-induced B-cell responses. (A) Effects of TACI siRNA and heparitinase treatment on B-cell proliferation (top), IgA production (middle), and IgG production (bottom) in response to CD40L, BAFF, APRIL, and agonistic anti-TACI mAb. IgA- or IgG-negative B cells treated with control siRNA, TACI siRNA, or heparitinase (10 U/mL) were cultured with anti-BCR antibodies (anti-Igκ and anti-Igλ, 0.5 μg/mL each), CD40L (2 μg/mL), BAFF (4 μg/mL), APRIL (8 μg/mL), anti-TACI mAb (11H3; 5 μg/mL) or control mouse IgG2a (5 μg/mL) in the presence or absence of IL-4 (20 U/mL) and TGF-β (1 ng/mL). [3H]-Thymidine incorporation in B cells was measured during the last 18 hours of 72-hour culture (top). IgA (middle) and IgG (bottom) secretion were measured by ELISA after 10-day culture. Data are mean ± SD. *P < .05. (B) Ratios of IgA/IgM and IgG/IgM production in response to CD40L, BAFF, APRIL, and agonistic anti-TACI mAb. After incubation, as described in panel A, IgM secretion and viable cell number were determined after 10-day culture (Table S1). Per cell IgA, IgG, or IgM production was calculated based on data shown in panel A and Table S1. Then the ratios of IgA/IgM and IgG/IgM were determined. Data are mean ± SD. *P < .05. (C) Flow cytometric analysis of IgA-positive B cells. After incubation as described in panel A, cells were stained with PE-labeled anti-CD19 mAb and FITC-conjugated anti-IgA antibody and were analyzed in living cells only. The percentage of CD19+ IgA+ cells is indicated in each plot. Data are representative of 3 independent experiments with similar results.
Effects of TACI siRNA and heparitinase on APRIL- and BAFF-induced B-cell responses. (A) Effects of TACI siRNA and heparitinase treatment on B-cell proliferation (top), IgA production (middle), and IgG production (bottom) in response to CD40L, BAFF, APRIL, and agonistic anti-TACI mAb. IgA- or IgG-negative B cells treated with control siRNA, TACI siRNA, or heparitinase (10 U/mL) were cultured with anti-BCR antibodies (anti-Igκ and anti-Igλ, 0.5 μg/mL each), CD40L (2 μg/mL), BAFF (4 μg/mL), APRIL (8 μg/mL), anti-TACI mAb (11H3; 5 μg/mL) or control mouse IgG2a (5 μg/mL) in the presence or absence of IL-4 (20 U/mL) and TGF-β (1 ng/mL). [3H]-Thymidine incorporation in B cells was measured during the last 18 hours of 72-hour culture (top). IgA (middle) and IgG (bottom) secretion were measured by ELISA after 10-day culture. Data are mean ± SD. *P < .05. (B) Ratios of IgA/IgM and IgG/IgM production in response to CD40L, BAFF, APRIL, and agonistic anti-TACI mAb. After incubation, as described in panel A, IgM secretion and viable cell number were determined after 10-day culture (Table S1). Per cell IgA, IgG, or IgM production was calculated based on data shown in panel A and Table S1. Then the ratios of IgA/IgM and IgG/IgM were determined. Data are mean ± SD. *P < .05. (C) Flow cytometric analysis of IgA-positive B cells. After incubation as described in panel A, cells were stained with PE-labeled anti-CD19 mAb and FITC-conjugated anti-IgA antibody and were analyzed in living cells only. The percentage of CD19+ IgA+ cells is indicated in each plot. Data are representative of 3 independent experiments with similar results.
TACI-dependent PKA activation is induced by APRIL but not by BAFF
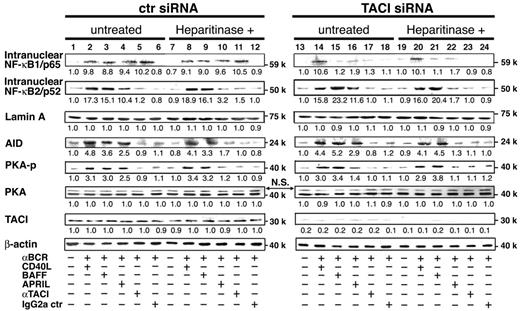
Nuclear factor-κ B (NF-κB) signaling has 2 pathways, canonical NF-κB1 and noncanonical NF-κB2 pathway, as evidenced by the nuclear translocation of p50/RelA (p65) and p52/RelB complexes,23,24 respectively. Stimulation with CD40L and BAFF preferentially activates the noncanonical pathway responsible for B-cell responses, such as cell proliferation, immunoglobulin production, and AID-mediated CSR.25 As shown in Figure 3, NF-κB1 translocation induced by BAFF, APRIL, and agonistic anti-TACI mAb (lanes 3-5) was greatly diminished by TACI siRNA (lanes 15-17) and by heparitinase treatment (lanes 21-23). Although NF-κB2 translocation was induced by BAFF and APRIL (lanes 3-4), the translocation by BAFF but not by APRIL was greatly enhanced by TACI siRNA (lane 15), which was irrelevant to the state of HSPG (lane 21). On the other hand, denaturation of HSPG diminished APRIL-induced NF-κB2 translocation (lane 4 vs lane 10), which was not influenced by TACI siRNA (lane 22 vs lane 16). Moreover, the induction of AID paralleled the behavior of NF-κB2 translocation, indicating that APRIL-induced NF-κB1 translocation is dependent on TACI and that APRIL/HSPG binding is necessary for NF-κB2 translocation and AID induction.
Effects of TACI siRNA and heparitinase on APRIL- and BAFF-induced NF-κB2 and AID expression and PKA phosphorylation. B cells pretreated with control siRNA, TACI siRNA, or heparitinase (10 U/mL) were cultured with anti-BCR antibodies (anti-Igκ and anti-Igλ, 0.5 μg/mL each), CD40L (2 μg/mL), BAFF (4 μg/mL), APRIL (8 μg/mL), anti-TACI mAb (11H3; 5 μg/mL), or control mouse IgG2a (5 μg/mL) in the presence of TGF-β (1 ng/mL). Incubation times differed with individual probes: 30 minutes for NF-κB1/p65 and TACI, 6 hours for NF-κB2/p52, 72 hours for AID, and 45 minutes for PKA phosphorylation. Nuclear extracts and cell lysates were prepared and subjected to immunoblot analysis. Density of each band was analyzed (LumiVision analyzer) and was presented as relative fold of the minimum density in each panel. Data are representative of 3 independent experiments with similar results.
Effects of TACI siRNA and heparitinase on APRIL- and BAFF-induced NF-κB2 and AID expression and PKA phosphorylation. B cells pretreated with control siRNA, TACI siRNA, or heparitinase (10 U/mL) were cultured with anti-BCR antibodies (anti-Igκ and anti-Igλ, 0.5 μg/mL each), CD40L (2 μg/mL), BAFF (4 μg/mL), APRIL (8 μg/mL), anti-TACI mAb (11H3; 5 μg/mL), or control mouse IgG2a (5 μg/mL) in the presence of TGF-β (1 ng/mL). Incubation times differed with individual probes: 30 minutes for NF-κB1/p65 and TACI, 6 hours for NF-κB2/p52, 72 hours for AID, and 45 minutes for PKA phosphorylation. Nuclear extracts and cell lysates were prepared and subjected to immunoblot analysis. Density of each band was analyzed (LumiVision analyzer) and was presented as relative fold of the minimum density in each panel. Data are representative of 3 independent experiments with similar results.
Next, we examined the status of PKA activation because PKA plays a critical role in the posttranslational regulation of AID activity and in the induction of immunoglobulin CSR through the phosphorylation of AID.26,27 PKA phosphorylation was clearly observed in response to CD40L, BAFF, and APRIL (lanes 2-4). When treated with heparitinase, PKA phosphorylation was blocked in response to APRIL but not to CD40L or BAFF (lanes 8-10). Loss of PKA phosphorylation in TACI knockdown B cells was observed in APRIL stimulation but not in BAFF (lanes 15-16). Thus, impaired APRIL-induced IgA secretion (Figure 2A) in TACI knockdown B cells correlated with impaired PKA phosphorylation but not AID induction or NF-κB2 translocation. These results suggest that APRIL-induced PKA activation requires TACI and HSPG ligation and that TACI may play a pivotal role in APRIL-induced IgA production by regulating AID activity through PKA phosphorylation.
Simultaneous coligation of TACI and HSPG is required for PKA activation and subsequent IgA production
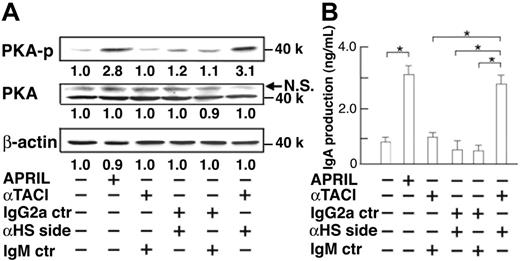
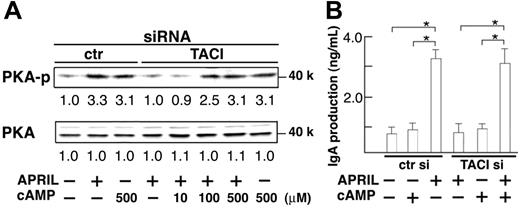
To confirm the mutual cooperation between TACI and HSPG in APRIL-induced IgA production, we tried to mimic the coligation of TACI and HSPG with specific antibodies and their second antibody instead of APRIL ligation. Figure 4 shows that phosphorylation of PKA (Figure 4A) and IgA secretion (Figure 4B) were induced when TACI and HSPG were coligated simultaneously, indicating that APRIL binding to TACI and HSPG is essential for IgA production. We then examined whether the defective PKA activation was sufficient to explain the lack of APRIL-induced IgA secretion in TACI knockdown B cells by testing whether constitutive PKA activity could compensate for the defect of TACI signaling. For constitutive activation of PKA, we used 8-bromo-cAMP, a cell-permeable cAMP analog known to act as a potent inducer of PKA activation.28 As expected, cAMP administration resulted in PKA phosphorylation even in the absence of TACI (Figure 5A), which in turn recovered APRIL-induced IgA secretion (Figure 5B), indicating the essential role of TACI-mediated PKA activation in APRIL-induced IgA production. Thus, HSPG-mediated AID induction was insufficient for IgA production and TACI-mediated PKA activation was indispensable for APRIL-induced IgA production. These results suggest that the defective secretion of IgA by TACI knockdown B cells was caused by insufficient PKA activation and that TACI and HSPG synergistically mediated a signal for PKA activation and IgA production.
Effects of coligation of TACI and HSPG on PKA phosphorylation and IgA production. (A) IgA-negative B cells were cultured with anti-TACI mAb (11H3), anti–heparan sulfate side chain mAb (10E4), mouse IgG2a, or mouse IgM (5 μg/mL each) for 45 minutes. Then anti–mouse Igκ (1 μg/mL) antibody was added to establish the cross-linking of cell-bound mAbs. APRIL (8 μg/mL) was used as a positive control. After 45-minute incubation, cell lysates were prepared and subjected to immunoblot analysis (B) IgA concentrations in culture supernatant were measured by ELISA after 10 days. Data are mean ± SD. *P < .05. Data are representative of 3 independent experiments with similar results.
Effects of coligation of TACI and HSPG on PKA phosphorylation and IgA production. (A) IgA-negative B cells were cultured with anti-TACI mAb (11H3), anti–heparan sulfate side chain mAb (10E4), mouse IgG2a, or mouse IgM (5 μg/mL each) for 45 minutes. Then anti–mouse Igκ (1 μg/mL) antibody was added to establish the cross-linking of cell-bound mAbs. APRIL (8 μg/mL) was used as a positive control. After 45-minute incubation, cell lysates were prepared and subjected to immunoblot analysis (B) IgA concentrations in culture supernatant were measured by ELISA after 10 days. Data are mean ± SD. *P < .05. Data are representative of 3 independent experiments with similar results.
Recovery of APRIL-induced IgA production by activation of PKA in the absence of TACI. Controller TACI siRNA-transfected B cells were cultured with different doses of cAMP analog 8-bromo-cAMP (A; 10, 100 or 500 μM) (B; 500 μM) for 2 hours. Cells were further cultured in the presence of APRIL (8 μg/mL), anti-BCR antibodies (anti-Igκ and anti-Igλ, 0.5 μg/mL each), and TGF-β (1 ng/mL). PKA phosphorylation by immunoblotting (A) or IgA secretion by ELISA (B) was evaluated after 45 minutes or 10 days culture, respectively. Data are mean ± SD. *P < .05. Data are representative of 3 independent experiments with similar results.
Recovery of APRIL-induced IgA production by activation of PKA in the absence of TACI. Controller TACI siRNA-transfected B cells were cultured with different doses of cAMP analog 8-bromo-cAMP (A; 10, 100 or 500 μM) (B; 500 μM) for 2 hours. Cells were further cultured in the presence of APRIL (8 μg/mL), anti-BCR antibodies (anti-Igκ and anti-Igλ, 0.5 μg/mL each), and TGF-β (1 ng/mL). PKA phosphorylation by immunoblotting (A) or IgA secretion by ELISA (B) was evaluated after 45 minutes or 10 days culture, respectively. Data are mean ± SD. *P < .05. Data are representative of 3 independent experiments with similar results.
Discussion
In the present study, we showed that TACI was a positive regulator for APRIL-induced IgA production and a negative regulator for BAFF-induced B-cell responses such as B-cell proliferation and production of IgA and IgG in human peripheral blood B cells in vitro. In addition, we demonstrated that HSPG was essential for APRIL-induced B-cell responses, including the activation of noncanonical NF-κB2 pathway and the induction of AID, whereas TACI contributed less to APRIL-induced B-cell responses except for PKA activation and IgA production.
The positive role of TACI and APRIL in IgA production appears to be important in vivo because TACI and APRIL knockout mice showed reduced levels of serum IgA and deficient IgA responses to mucosally immunized antigens, and old APRIL transgenic mice showed increased levels of serum IgA.13,14,19,29,30 In addition, defects in TACI have been reported in patients with IgA deficiency and CVID.16,17 Why is APRIL/TACI rather than BAFF/BAFF-R dominant in IgA production in vivo? The reason might be explained by the localization of IgA-producing cells and TACI-expressing cells. Most IgA antibody is synthesized by mucosal plasma cells associated with lymphoid tissue, gut-associated B cells, and B-1 B cells.31,32 It has been reported that TACI but not BAFF-R is highly expressed in human small intestine3,7 and that murine B-1 B cells bind strongly and specifically to APRIL.30
We observed that TACI stimulation itself could not induce B-cell activation (Figure 2A); thus, it is unlikely that TACI alone is involved directly in APRIL-induced IgA production. In addition, a previous report described a deficiency of immunoglobulin CSR in TACI knockout B cells after stimulation with APRIL in mice,29 which is almost similar to the result described here for IgA production with TACI knockdown, in addition to the up-regulation of AID and the mild reduction in IgG. These observations suggest that HSPG plays a major role in APRIL-induced immunoglobulin CSR and that the synergistic ability of TACI is responsible for IgA CSR with HSPG. In this regard, we demonstrated that the coligation of TACI and HSPG by specific mAbs clearly induced IgA production instead of APRIL (Figure 4). However, because HSPG is widely expressed throughout the B-cell lineage as transmembrane proteins such as CD44 and syndecan 18,33 and the expression of TACI varies after activation,34 TACI seems to play a critical role in APRIL-induced IgA production under physiological conditions.
We showed here that although AID was expressed at comparable levels in B cells with TACI and control siRNA (Figure 3), APRIL-induced IgA secretion was impaired by TACI siRNA whereas IgG secretion was slightly reduced compared with siRNA control (Figure 2). It is possible that the dissimilarity in TACI-mediated immunoglobulin responses resulted from differences in the threshold of PKA-mediated phosphorylation that affect AID functionality to induce immunoglobulin CSR. Castigli et al29 demonstrated the preferential induction of molecular events in IgG CSR rather than IgA CSR in TACI knockout B cells in response to APRIL. Basu et al26 identified several phosphorylation sites on AID responsible for IgG CSR and demonstrated marked abrogation of IL-4–, TGF-β–, and CD40-induced IgA CSR by PKA inhibition, suggesting that IgA CSR might require more potent PKA activity than IgG CSR. Our finding that activated PKA by a cAMP analog has a capacity sufficient to induce APRIL-induced IgA secretion, despite the absence of TACI (Figure 5), suggests that AID is induced by the binding of APRIL to HSPG and becomes activated after TACI-dependent proper regulation of PKA.
It has been reported that mouse splenic B cells proliferate in response to a form of APRIL that cannot bind HSPG or APRIL in the presence of heparin.9 However, we could not observe APRIL-induced proliferation of human peripheral blood B cells treated with heparitinase (Figure 2A) and cultured in the presence of heparin (data not shown). Perhaps these results were different because the APRIL splice variant present in mice can bind to mouse BAFF-R though it does not exist in humans and because human APRIL does not interact with human BAFF-R, as reported recently.35 In addition, it has been reported that TACI knockout B cells do not show IgA CSR and production in response to BAFF in mice.29 Given that we could not observe an obvious defect in IgA secretion by TACI knockdown B cells in response to BAFF, the different results might be attributed, at least in part, to differences between murine splenic B cells and human peripheral blood B cells.
Although normal humoral responses to TD-Ags and B-cell maturation have been observed in TACI knockout mice, humoral responses to TI-Ags were impaired.13 B cells responding to TI-Ags reside largely in marginal zone (MZ) B-cell and B-1 B-cell compartments.36,37 It has been reported that TACI is highly expressed on mouse MZ B cells34 and that B-1 B cells bind strongly and specifically to APRIL.30 Thus, it is possible that adequate APRIL binding to TACI and HSPG on these B cells is a more likely explanation of the effects observed in TACI knockout mice. MZ B cells and B-1 B cells can readily undergo CSR from IgM to IgG or IgA in a T cell–independent fashion.38 Recent studies suggest that T cell–independent CSR requires the interaction of B cells with dendritic cells.21,39 APRIL secreted from dendritic cells by TI-Ags might enable proper antibody production from MZ and B-1 B cells.
Homozygous and heterozygous mutations in TNFRSF13B are associated with the loss of TACI function, as reported in patients with CVID and IgA deficiency.16,17 Conversely, strong induction of mature B-cell proliferation, B-cell hyperresponsiveness, lymphadenopathy, and systemic lupus erythematosus nephritis was observed in TACI knockout mice.13-15 However, only a minority of CVID patients showed signs of autoimmunity or lymphoproliferation.16 In theory, it could still be possible that HSPG or TACI is expressed on human B cells at levels different from those on murine B cells, reflecting differences in severity. As discussed, TACI is a negative regulator for BAFF-induced B-cell responses and a positive regulator of APRIL-induced IgA production and humoral responses to TI-Ags in collaboration with HSPG. Thus, immunoglobulin deficiency other than IgA observed in CVID patients with TACI defects might have resulted from other CVID disease-specific factors.
In conclusion, the present study identified bilateral regulatory roles of TACI B-cell responses and their importance, especially in IgA production in collaboration with HSPG. These new findings should enhance our understanding of mucosal immune system and humoral responses to TI-Ags.
Authorship
Contribution: D.S. performed the experiments and wrote the paper; H.H. analyzed the data and participated in the writing of the paper; Y.K. collected the data; H.K. analyzed the data; K.O. contributed vital new reagents; and T.K. designed the research protocol and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tetsuji Kobata, Department of Immunology, Dokkyo Medical University School of Medicine, 880 Kitakobayashi, Mibu, Tochigi 321-0293, Japan; tkobata@dokkyomed.ac.jp.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by Dokkyo University School of Medicine Investigator-Initiated Research grant 2005-01-8 (D.S.); a grant for Hi-Tech Research from Dokkyo Medical University School of Medicine (T.K.); Grant-in-Aid for Scientific Research (C) KAKENHI 16590410 (T.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Health and Labour Sciences Research Grants for Research on Health Sciences focusing on Drug Innovation from the Ministry of Health, Labour, and Welfare of Japan (T.K.); and the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan (T.K.).
We thank Dr H. Karasuyama for the pBCMGSneo expression vector, Dr S. F. Schlossman for helpful discussion, and Y. Nitta for secretarial assistance. We also thank the Laboratory Animal Research Center and Laboratory of Analytical Instruments, Institute for Medical Science, Dokkyo Medical University, for the use of their facilities and the Japanese Red Cross Tochigi Blood Center for leukopaks. We thank Dr F. G. Issa for the critical reading of the manuscript.

![Figure 2. Effects of TACI siRNA and heparitinase on APRIL- and BAFF-induced B-cell responses. (A) Effects of TACI siRNA and heparitinase treatment on B-cell proliferation (top), IgA production (middle), and IgG production (bottom) in response to CD40L, BAFF, APRIL, and agonistic anti-TACI mAb. IgA- or IgG-negative B cells treated with control siRNA, TACI siRNA, or heparitinase (10 U/mL) were cultured with anti-BCR antibodies (anti-Igκ and anti-Igλ, 0.5 μg/mL each), CD40L (2 μg/mL), BAFF (4 μg/mL), APRIL (8 μg/mL), anti-TACI mAb (11H3; 5 μg/mL) or control mouse IgG2a (5 μg/mL) in the presence or absence of IL-4 (20 U/mL) and TGF-β (1 ng/mL). [3H]-Thymidine incorporation in B cells was measured during the last 18 hours of 72-hour culture (top). IgA (middle) and IgG (bottom) secretion were measured by ELISA after 10-day culture. Data are mean ± SD. *P < .05. (B) Ratios of IgA/IgM and IgG/IgM production in response to CD40L, BAFF, APRIL, and agonistic anti-TACI mAb. After incubation, as described in panel A, IgM secretion and viable cell number were determined after 10-day culture (Table S1). Per cell IgA, IgG, or IgM production was calculated based on data shown in panel A and Table S1. Then the ratios of IgA/IgM and IgG/IgM were determined. Data are mean ± SD. *P < .05. (C) Flow cytometric analysis of IgA-positive B cells. After incubation as described in panel A, cells were stained with PE-labeled anti-CD19 mAb and FITC-conjugated anti-IgA antibody and were analyzed in living cells only. The percentage of CD19+ IgA+ cells is indicated in each plot. Data are representative of 3 independent experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/7/10.1182_blood-2006-08-041772/4/m_zh80070710140002.jpeg?Expires=1767802338&Signature=KzOOHg7fOrNodmnsBFBskPsLjnCmcmw8eGULZOvv5xHLiquUdxa61XZs9efS~YHjU7JAp0rBrmFQLvbq-Dhnha8TWrvfYx5XoLwUc9ynocZ7oKyXBizoAXR5NLsx8urpnFcg9cPTnawrtq-RSSMuozcTDhWPh1Qy22KkNqVTrrORwdL9kmEhge2MNWuirP4HDOKHqTxN1Ico1IijOyNPSRVKo1kuXnLUBmCMkY0HyhLXcB4ScsMrmEoTckrlVw~LvrHiBTA6UEfvsMfroCoNaxp0LMdtYAq9DntpLo~vdKqlIMAd5STVhZYefcyx-1JyIAtHYbtAYTbyvXWvD27qyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)