Abstract
Adoptive T-cell therapy in cancer or chronic viral infections is often impeded by the development of functional impairment of the transferred cells. To overcome this therapeutic limitation we combined adoptive transfer of naive, virus-specific CD8+ T cells with immunostimulative CpG oligodeoxynucleotides (ODNs) in mice chronically infected with the Friend retrovirus. The CpG-ODN co-injection prevented the T cells from developing functional defects in IFNγ and granzyme production and degranulation of cytotoxic molecules. Thus, the transferred T cells were able to reduce chronic viral loads when combined with CpG-ODNs. This strategy provides a new approach for developing successful adoptive T-cell therapy against chronic infections.
Introduction
T cells recognize tumors or infected cells and prevent onset of disease by killing these target cells. However, the interplay of tumors or pathogens and the immune system is complex, as demonstrated by cancer or chronic infections developing in the presence of specific T cells, whereby the pathogens or tumors obviously could evade T-cell surveillance. One strategy for fighting chronic infections or aggressive cancer is adoptive T-cell therapy, which involves the transfer of effector T cells to restore specific T-cell responses in the host. Recent technical developments to obtain T cells of wanted specificities have created increasing interest in using adoptive T-cell therapy in different clinical settings. However, although some successful clinical trials for cancer therapy1 or treatment of chronic infections2 were conducted, the loss of biological function of transferred T cells within the recipient remains a serious problem. Frequently, T-cell–mediated responses were impeded by the development of a functional impairment of the transferred cells.3,4 Obviously, adoptively transferred cells are functionally impaired by similar mechanisms that lead to immune escape from the endogenous T-cell response. In this study, we used the Friend retrovirus (FV) mouse model to develop a strategy of adoptive T-cell therapy that allows the transferred cells to maintain their functional properties in a chronically infected host.
Friend retrovirus (FV) is a viral complex comprising 2 retroviruses5 that induces lethal leukemia in susceptible mice, but resistant strains recover from acute disease. However, these mice are not able to completely eradicate the virus, and a chronic infection develops.6 Like in other chronic virus infections,7,8 CD8+ T cells are functionally impaired by regulatory T cells during persistent FV infection,9 and we could show that adoptively transferred virus-specific CD8+ T cells also lose their ability to kill infected target cells in chronically infected mice.10 As a consequence, adoptive CD8+ T cell transfers did not have any therapeutic effect on virus loads. To overcome this limitation we injected virus-specific T cells together with synthetic oligodeoxynucleotides containing immunostimulatory CpG motifs (CpG-ODNs). CpG-ODNs can activate antigen-presenting cells in vivo and subsequently augment specific T-cell responses.11
Materials and methods
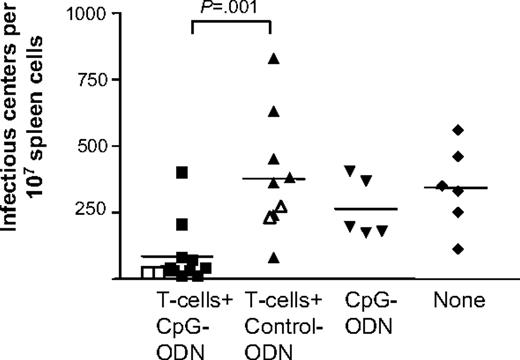
Resistant mice were infected with FV; 8 weeks later, these mice had developed a chronic infection with stable virus loads (Figure 1). For adoptive T-cell therapy, naive, virus-specific CD8+ T cells from B6 mice carrying a transgenic T-cell receptor (TCRtg) specific for the H-2Db–restricted gagL epitope of FV were used.13 The T cells were genetically labeled (CD45.1) to distinguish between host and donor cells. As expected, the intravenous injection of 4 × 106 TCRtg T cells could not reduce virus loads in chronically infected mice (Figure 1).10 To overcome their functional impairment, we coinjected CpG-ODNs. Using different treatment protocols, 15 nmol CpG-ODNs were injected twice on days 0 and 4, or alternatively once on day 2 or on day 5 after cell transfer.
Virus load after adoptive transfer of virus-specific CD8+ T cells. Chronically FV-infected (B10 × A.BY) F1 mice were treated with 4 × 106 naive, virus-specific TCRtg CD8+ T cells and 15 nmol of the B-type CpG-ODN (CpG-1668: 5′-tcc atg acg ttc ctg atg ct-3′) (CpG) or control ODN without the CpG motif (5′-tcc atg agc ttc ctg atg ct-3′)12 (Control) were injected twice, once on the day of T-cell transfer and again 4 days later. As controls, 1 group of animals received CpG-ODNs without T cells and 1 group of chronically infected mice did not receive any treatment (none). At 1 week (closed symbols) or 3 weeks (open symbols) after transfer, the spleen cells of the recipients were harvested and virus loads were determined in an infectious center assay.9 Mean values for each group are indicated by bars. The differences in viral loads between the groups of mice receiving CpG-ODNs or control ODNs together with T cells were statistically significant (unpaired t test).
Virus load after adoptive transfer of virus-specific CD8+ T cells. Chronically FV-infected (B10 × A.BY) F1 mice were treated with 4 × 106 naive, virus-specific TCRtg CD8+ T cells and 15 nmol of the B-type CpG-ODN (CpG-1668: 5′-tcc atg acg ttc ctg atg ct-3′) (CpG) or control ODN without the CpG motif (5′-tcc atg agc ttc ctg atg ct-3′)12 (Control) were injected twice, once on the day of T-cell transfer and again 4 days later. As controls, 1 group of animals received CpG-ODNs without T cells and 1 group of chronically infected mice did not receive any treatment (none). At 1 week (closed symbols) or 3 weeks (open symbols) after transfer, the spleen cells of the recipients were harvested and virus loads were determined in an infectious center assay.9 Mean values for each group are indicated by bars. The differences in viral loads between the groups of mice receiving CpG-ODNs or control ODNs together with T cells were statistically significant (unpaired t test).
Results and discussion
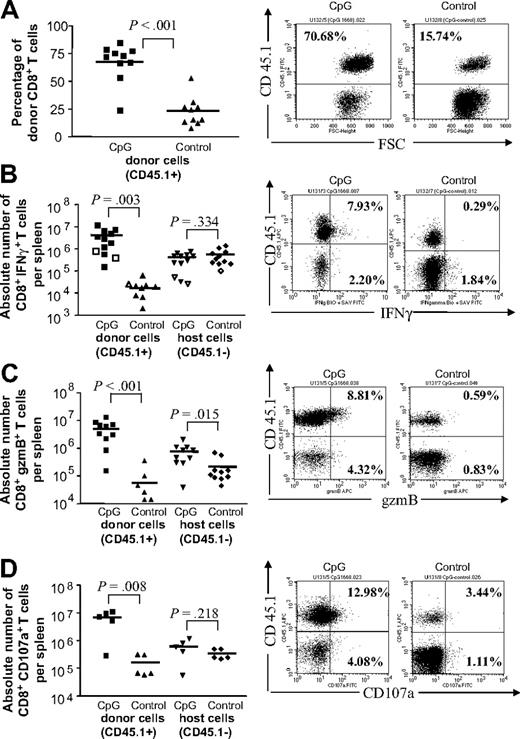
A week after early therapy with CpG-ODNs (day 0/4 or 2), the percentage of CD45.1+ donor CD8+ T cells in the spleen was nearly 3 times higher in mice coinjected with CpG-ODN type 1668 than in animals that had received T cells plus a control ODN without a CpG motif (Figure 2A; Supplemental Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, the antiviral activity of the donor cells had also significantly improved.
Production of IFNγ and granzyme B and detection of exocytosis in adoptively transferred CD8+ T cells. Chronically FV-infected (B10 × A.BY) F1 mice were treated with 4 × 106 naive, virus-specific CD8+ T cells from B6 mice carrying a TCRtg specific for the H-2Db–restricted gagL epitope of FV. The transferred CD8+ T cells were genetically labeled (CD45.1) to distinguish the host cell population from the donor cell population. B-type CpG-ODN 1668 (CpG) or control ODN without CpG motif (Control) (15 nmol) were injected twice. At 1 week after transfer, spleen cells of the recipient were harvested, and host (CD45.1−) and donor (CD45.1+) CD8+ T cells were stained for granzyme B (gzmB), IFNγ, and CD107a.9 Accumulated results are shown in the left column, with representative flow data in the right column. (A) The difference in the percentages of CD8+ donor T cells (CD45.1+) was statistically significant between the mice receiving CpG-ODNs or control ODNs. (B) Harvested cells were restimulated for 5 hours in vitro with α-CD3 and α-CD28 (closed symbols) or with 5 μg/mL FV DbGagL peptide and α-CD28 (open symbols) to detect intracellular IFNγ. Nonstimulated CD45.1+ CD8+ cells showed a background of less than 0.05% IFNγ-positive cells. The difference in absolute numbers of CD8+ donor (CD45+) T cells (CD45.1–) producing IFNγ was statistically significant between the mice receiving CpG-ODNs or control ODNs. (C) Harvested cells were analyzed directly ex vivo to detect intracellular granzyme B. The difference in absolute numbers of CD8+ donor and host T cells (CD45.1+) producing granzyme B was statistically significant between the mice receiving CpG-ODNs or control ODNs. Similar results were obtained for granzyme A production by CD8+ T cells (data not shown). (D) Harvested cells were analyzed directly ex vivo to detect CD107a expression. The difference in absolute numbers of CD8+ donor T cells (CD45.1+) expressing CD107a was statistically significant between the mice receiving CpG-ODNs or control ODNs. Percentages of positive donor (top right) and host (bottom right) cells are given in the respective quadrants. Mean values for each group are indicated by bars. Statistical analyses were done by unpaired t test.
Production of IFNγ and granzyme B and detection of exocytosis in adoptively transferred CD8+ T cells. Chronically FV-infected (B10 × A.BY) F1 mice were treated with 4 × 106 naive, virus-specific CD8+ T cells from B6 mice carrying a TCRtg specific for the H-2Db–restricted gagL epitope of FV. The transferred CD8+ T cells were genetically labeled (CD45.1) to distinguish the host cell population from the donor cell population. B-type CpG-ODN 1668 (CpG) or control ODN without CpG motif (Control) (15 nmol) were injected twice. At 1 week after transfer, spleen cells of the recipient were harvested, and host (CD45.1−) and donor (CD45.1+) CD8+ T cells were stained for granzyme B (gzmB), IFNγ, and CD107a.9 Accumulated results are shown in the left column, with representative flow data in the right column. (A) The difference in the percentages of CD8+ donor T cells (CD45.1+) was statistically significant between the mice receiving CpG-ODNs or control ODNs. (B) Harvested cells were restimulated for 5 hours in vitro with α-CD3 and α-CD28 (closed symbols) or with 5 μg/mL FV DbGagL peptide and α-CD28 (open symbols) to detect intracellular IFNγ. Nonstimulated CD45.1+ CD8+ cells showed a background of less than 0.05% IFNγ-positive cells. The difference in absolute numbers of CD8+ donor (CD45+) T cells (CD45.1–) producing IFNγ was statistically significant between the mice receiving CpG-ODNs or control ODNs. (C) Harvested cells were analyzed directly ex vivo to detect intracellular granzyme B. The difference in absolute numbers of CD8+ donor and host T cells (CD45.1+) producing granzyme B was statistically significant between the mice receiving CpG-ODNs or control ODNs. Similar results were obtained for granzyme A production by CD8+ T cells (data not shown). (D) Harvested cells were analyzed directly ex vivo to detect CD107a expression. The difference in absolute numbers of CD8+ donor T cells (CD45.1+) expressing CD107a was statistically significant between the mice receiving CpG-ODNs or control ODNs. Percentages of positive donor (top right) and host (bottom right) cells are given in the respective quadrants. Mean values for each group are indicated by bars. Statistical analyses were done by unpaired t test.
Both IFNγ and cytotoxic granzymes have been shown to be critical for control of acute FV replication,14,15 but they are suppressed during chronic infection.9,10 The percentage of virus-specific donor T cells producing IFNγ was significantly increased when CpG-ODNs were coinjected compared with control ODN coinjection (Figure 2B). In absolute numbers of IFNγ-producing cells per spleen, the mean difference between both groups was more than 240-fold. Similar findings were made for the cytotoxic response of the transferred T cells. Granzymes mediate cytotoxic activity in acute FV infection,14 but in chronically infected mice, most adoptively transferred CD8+ T cells were unable to produce granzymes (Figure 2C). This defect could also be overcome by coinjecting CpG-ODNs early after T-cell transfer, as the percentages and absolute numbers of granzyme B (Figure 2C) producing CD45.1+ donor T cells increased significantly. The cytotoxic activity of donor CD8+ T cells was further analyzed by examining cell-surface expression of the degranulation marker CD107a,16 which can be used a surrogate marker for FV-specific T-cell killing.9 CD107a expression is significantly enhanced on CD45.1+ cells that were cotransferred with CpG-ODNs early after cell transfer when compared with control ODNs (Figure 2D), with the absolute numbers of CD107a+ killer cells being more than 40 times higher in the CpG-ODN group. In contrast, animals that received CpG-ODNs 5 days after cell transfer showed much weaker expansion of the donor cell population, and the function of these cells was only slightly improved compared with controls (Figure S1).
Interestingly, the CpG-ODN treatment had only minor effects on the endogenous CD45.1−CD8+ T-cell population, as only the numbers of granzyme B–producing T cells were significantly increased after CpG-ODN inoculation (Figure 2C). This was consistent with findings that CpG-ODNs or virus-specific CD8+ T cells alone could not reduce virus loads in chronically infected mice (Figure 1). In contrast, a combination of T cells plus CpG-ODNs on days 0 and 4, or 2 days after cell transfer, could significantly reduce chronic viral loads 1 week after infection (Figure 1; data not shown), which correlated with the improved functional properties of the donor T cells. A similar reduction in viral load was still found at 3 weeks after transfer (Figure 1), when activation of donor T cells was no longer detectable. However, late treatment with CpG-ODNs at 5 days after transfer did not result in reduced viral loads (data not shown), indicating that CpG-ODNs have to be present during the first few days after transfer to allow for successful T-cell therapy.
Thus, adoptive T-cell therapy using naive or memory T cells might be a feasible approach for treating chronic viral infections if combined with CpG-ODN administration. However, adoptive T-cell transfers with T cells that were expanded in vitro did not result in successful treatment of chronic infection. Such preactivated cells developed functional impairments after transfer and did not reduce FV loads regardless of whether they were cotransferred with CpG-ODNs or not (data not shown). The efficacy of naive T cells might reflect distinct mechanisms of activation upon an encounter with antigen. The naive virus-specific T cells required for therapy could potentially be generated using TCR transfer technology,4,17,18 a method currently being pursued clinically.19 To accomplish this, naive T cells could be isolated from chronically infected patients and transduced with lentiviral vectors containing TCRs that target selective epitopes of the virus. Our data imply that such cells would expand very efficiently when cotransferred with CpG-ODNs, suggesting that only limited numbers of cells might be needed for effective therapy, which could improve the current therapy of chronic viral infections. In leukemia treatment it has been shown that immune tolerance of adoptively transferred T cells can be overcome by interleukin-15 (IL-15) treatment of the cells in vitro, which rescued antitumor activity in vivo.1 IL-15 directly stimulated the transferred CD8+ T cells that expressed the IL-15 receptor. This mechanism is unlikely for CpG-ODN treatment, since T cells do not express the receptor for CpG-ODN, toll-like receptor-9.20 This is mainly expressed by dendritic cells (DCs), which are activated upon CpG-ODN treatment.21 Thus, the activation of donor CD8+ T cells after transfer should be an indirect CpG-ODN effect through DC stimulation. Indeed, it has been shown that DC stimulation with CpG-ODNs can induce enhanced proliferation and survival of adoptively transferred T cells.22 We found that DCs can be infected by FV, which impaired their ability to mature (data not shown). Infection of DCs has been reported for many other viruses and might be a reason why adoptive T-cell therapy is often ineffective in chronic infections. CpG-ODN treatment of FV-infected DCs could partly overcome the virus-induced block in DC maturation (data not shown). Furthermore, in FV-infected DC cultures with naive TCRtg CD8+ T cells, coadministration of CpG-ODNs could augment functional properties of T cells (Table S2), indicating that the CpG-ODN treatment activated infected DCs, which resulted in stimulation of FV-specific CD8+ T cells. Here, the coadministration of T cells and CpG-ODNs induced a significant and sustained reduction of persistent viral loads but did not lead to the ultimate goal of complete viral clearance. One reason for incomplete virus clearance might be that regulatory T cells that were not affected by the CpG treatment (data not shown) were able to partly suppress the function of transferred T cells. Another alternative could be the treatment with a clonotypic CD8+ T-cell population that could allow the virus to partly escape immune control. Thus, a combination therapy with CpG-ODNs plus T cells of more than 1 specificity might augment the antiviral effect.
In summary, CpG-ODNs can effectively improve adoptive T-cell therapy if the transferred cells require in vivo priming by DCs. This is the case for naive T cells and may also be applicable for boosting memory T-cell responses, as studies suggest that activating DCs with CpG-ODNs during boosting will enhance CD8 memory responses to viruses.23 Thus, CpG-ODNs might also improve adoptive T-cell therapy using resting memory T-cells that could be isolated by major histocompatibility complex (MHC) multimer technology.24 This approach of transferring resting T cells with desired specificities that can then be efficiently expanded and functionally activated by CpG-ODNs in vivo needs to be investigated further in different disease models.
Authorship
Contribution: F.K. and S.S. performed research and analyzed data; C.O. contributed vital new reagents; P.D.G. contributed vital new reagents and wrote parts of the paper; and A.R.M.K. and U.D. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulf Dittmer, Institut für Virologie, Universität Duisburg-Essen, Hufelandstrasse 55, 45122 Essen, Germany; e-mail: ulf.dittmer@uni-due.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants to U.D. from the Deutsche Forschungsgemeinschaft (Di 714/6-2 and in part Di 714/7-2 and Di 714/6-3).
The current address of A.R.M.K. is: Department of Pathology, University of Massachusetts Medical School, Worcester, MA.