Abstract
CD10 and MUM1 are representative B cell differentiation markers. Follicular lymphoma (FL) is usually positive for CD10 and negative for MUM1. In this study, however, we compared 22 FLs with peculiar phenotype CD10−MUM1+ with 119 typical CD10+MUM1− FLs. All CD10−MUM1+ FL patients exhibited follicular structure with follicular dendritic meshwork, and a high rate of somatic hypermutation and ongoing mutation, similar to typical FL. However, CD10−MUM1+ FLs were encountered frequently in the elderly compared with CD10+MUM1− typical FLs (67.0 versus 58.7 years, P < .01), showed high grade (grade 3A or 3B) morphology (91% versus 17%, P < .001), diffuse proliferation (59% vs 19%, P < .001), and lacked BCL2/IGH translocation (5% versus 92.5%, P < .001), which is the most characteristic aberration in FL, and 88% showed BCL6 gene abnormalities (translocation or amplification). Our results indicate that CD10−MUM1+ FL is different from typical FL with respect to biologic and clinical features.
Introduction
Follicular lymphoma (FL) is the most prevalent form of low-grade B-cell lymphoma in adults.1 Typically, FL cells express CD10, BCL2, and BCL6. CD10 is a marker for germinal center (GC) B cells, and thus its expression suggests that GC B cells are a normal counterpart of FL.2 However, some reports, including our previous study, described the existence of CD10− FL, especially in high-grade (grade 3) FL.3-6 However, it is not clear whether CD10 negativity is just aberrant loss or whether it is meaningful, reflecting a specific differentiation stage and affecting clinical features. MUM1 (multiple myeloma oncogene 1)/IRF4 (interferon regulatory factor 4) is a lymphoid-specific member of the interferon regulatory factor family of transcription factors,7-9 and it is a reliable marker of “late-stage GC” or “post-GC” B cells.8 In this study, we clinicopathologically compared CD10− MUM+ and “classical” CD10+MUM1− FLs
Materials and methods
Biologic material
Tissue specimens were obtained from human lymph nodes filed at the Department of Pathology at Fukuoka University and Kurume University. The 147 FL patients have already been reported in our previous publication.5 Paraffin-embedded tissues were available in almost all patients, while frozen tissues and cell suspensions were available in some patients. Histopathological diagnoses and grading were based on the new WHO classification and carried out by 4 pathologists (Y.G., K.K., M.K., and K.O.).1 Clinical information was obtained by reviewing the tumor registry records and/or patients' medical charts. This study was approved by the Kurume University institutional review board (Kurume, Japan), and patients provided informed consent in accordance with the Declaration of Helsinki.
Immunohistochemistry
Paraffin sections from each sample were immunostained with monoclonal antibodies against CD10 (Novocastra, Newcastle, United Kingdom), Bcl2 (DAKO, Glostrup, Denmark), MUM1 (DAKO), CD21 (DAKO), CD138 (Novocastra), and Bcl6 (Novocastra) following the method described previously.5 The following 2 categories were defined: negative (< 30% positively-stained tumor cells) and positive (≥ 30% positively-stained tumor cells).
Fluorescence in situ hybridization (FISH)
We used 39 cell suspensions fixed with methanol–acetic acid (3:1) and 4 paraffin-embedded tissue blocks. We used LSI IgH Spectrum Green/LSI Bcl2 Spectrum Orange Dual-Fusion Translocation Probe (Vysis, Downers Grove, IL) and the LSI Bcl6 Dual Color breakpoint probe (Vysis). Analyses were performed using the methods described previously.5 We were able to evaluate gene translocation as well as amplification in patient samples fixed with methanol–acetic acid (3:1). However, we could not evaluate gene amplification in paraffin-embedded tissues because morphologic detection of single cells was difficult in these samples.
Somatic mutation analysis of VH genes
DNA was prepared from whole sections of 10 paraffin-embedded tissues. Somatic mutations and ongoing mutations in immunoglobulin VH region were identified by using primer sets (FR2B, SJHa, and SJHb) and methods described previously.10
Statistical analysis
We used Student t test and chi-square test to compare clinical and pathological findings among different groups. A P value less than .05 denoted the presence of a statistically significant difference.
Results and discussion
Clinical features of CD10−MUM1+ FL
We compared the clinical features of patients with CD10−MUM1+ FL and 131 patients with CD10+MUM1− FL (Table 1; Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). CD10−MUM1+ FL patients were predominantly elderly men compared with CD10+MUM1− FL patients (males: 73% vs 41%, age: 67 vs 58.7 years, respectively). The prognosis of patients with CD10−MUM1+ is summarized in Table S1. Although the follow-up duration was relatively short in this study (6-72 months; median follow-up: 13 months), only 2 patients survived longer than 5 years. Despite the small number of patients and the short duration of follow-up, CD10−MUM1+ FL patients showed relatively poor prognosis in this study. MUM1 is expressed in various B-cell lymphomas and its expression is associated with clinical features, including prognosis, especially in diffuse large B-cell lymphoma (DLBCL).11-13 Further clinical studies that include a larger number of samples and longer follow-up period are needed to clarify the impact of MUM1 expression on the prognosis of FL.
Pathological features of CD10−MUM1+ FL and comparison with CD10+MUM1−FL
Among 21 CD10− FL patients that have been analyzed previously,5 8 (38%) were MUM1+, while only 1 patient (1.3%) among 83 CD10+ patients was MUM1+. Thus, it is conceivable that MUM1 expression in FL is linked to CD10 negativity. We added 14 CD10−MUM1+ newly diagnosed FL patients, and a total of 22 CD10−MUM1+ FL patients were analyzed and compared with CD10+MUM1− typical FL (Table 1 and Figure 1; Table S1). CD10−MUM1+ FL showed high-grade (grade 3A or 3B) morphology compared with CD10+MUM1− typical FL (91% vs 17%, P < .001). CD10−MUM1+ FL showed more frequent diffuse proliferation (59% vs 19%, P < .001) and less frequent BCL2 protein expression (59% vs 93.7%, P < .001) and lacked BCL2/IGH translocation (5% vs 92.5%, P < .001), compared with typical FL. BCL6 gene abnormalities (translocation and amplification) were identified in 88% (15/17) of CD10−MUM1+ FLs. Staining for CD138 was negative, and CD21 staining showed follicular dendritic cell meshwork in all CD10−MUM1+ FLs (Figure 1B). There was no clear difference between grade 3B and 2/3A among CD10−MUM1+ FLs (Table S1). The frequency of somatic mutation of immunoglobulin heavy chain in CD10−MUM1+ FLs ranged from 2.04% to 25.69%, with a mean of 11.81%. Intraclonal diversity, which reflects ongoing mutation, was detected in all patients, suggesting definitive germinal center B-cell origin (Table S2). Since CD10+MUM1− FLs are mainly of low-grade (grade 1/2) type, we selected 20 patients of high-grade (grade 3A/3B) CD10−MUM1+ FL and 20 patients of CD10+MUM1− FL to exclude bias based on morphologic differences. For analysis of BCL6 gene abnormality, we added 23 new CD10+MUM1− high-grade patients. However, CD10−MUM1+ FL still lacked BCL2/IGH gene translocation and showed BCL6 gene abnormality, while BCL2 protein expression was not different relative to typical FL. Furthermore, to validate MUM1 expression as a marker in CD10− FL, we compared CD10−MUM1+ with CD10−MUM1− FL (n = 30). In the latter group, only 44% (13/30) showed high-grade morphology (grade 3) (P < .01, vs CD10−MUM1+ FL). Similarly, 50% (13/26) (P < .01) and 36% (11/30) (P = .11) lacked BCL2 gene rearrangement and combined diffuse proliferation area, respectively. These proportions were lower than CD10−MUM1+ FL. These results suggest that MUM1 expression defines more homogeneous disease entity among CD10− FLs.
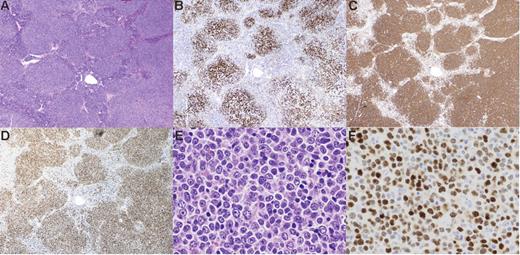
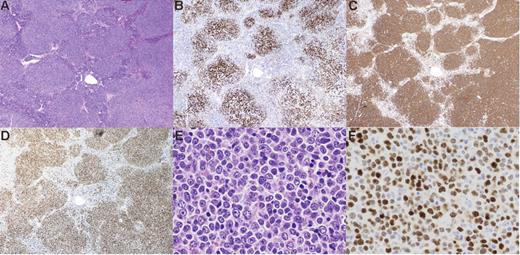
Pathological features of CD10−MUM1+ follicular lymphoma. In H&E sections, the lymphoma cells form definite follicular structures (A). The follicular structures are confirmed by follicular dendritic cell (FDC) meshwork stained for CD21 (B). In this case, almost all lymphoma cells are large cells and their proliferation shows a “sheet-like” pattern (E). This patient was diagnosed as grade 3B. Lymphoma cells are positive for CD20 (C) and MUM1 (D,F). All figures are from patient 7. Original magnification: (A-D) × 10, (E-F) × 100. Images were visualized using an Olympus AX80 microscope (Olympus, Tokyo, Japan), equipped with (A-D) an Olympus Planapo 4×/0.16 numerical aperture (NA) objective or (E-F) an Olympus Planapo 40×/0.95 NA objective. Images were captured using an Olympus DP70 camera and Olympus DP controller software, and were processed using Olympus DP manager software.
Pathological features of CD10−MUM1+ follicular lymphoma. In H&E sections, the lymphoma cells form definite follicular structures (A). The follicular structures are confirmed by follicular dendritic cell (FDC) meshwork stained for CD21 (B). In this case, almost all lymphoma cells are large cells and their proliferation shows a “sheet-like” pattern (E). This patient was diagnosed as grade 3B. Lymphoma cells are positive for CD20 (C) and MUM1 (D,F). All figures are from patient 7. Original magnification: (A-D) × 10, (E-F) × 100. Images were visualized using an Olympus AX80 microscope (Olympus, Tokyo, Japan), equipped with (A-D) an Olympus Planapo 4×/0.16 numerical aperture (NA) objective or (E-F) an Olympus Planapo 40×/0.95 NA objective. Images were captured using an Olympus DP70 camera and Olympus DP controller software, and were processed using Olympus DP manager software.
Until now, several reports have described an “atypical” minority subtype of FL.3-6,14-16 Although this group is different from typical FL, it is still a heterogeneous disease entity. High-grade FL, especially grade 3B, has been discussed as a different entity from typical low-grade FL because of the low-rate CD10 expression and BCL2 gene translocation and high-rate BCL6 gene translocation.3,6,15 However, the proportion of CD10+ patients among grade 3B FLs varied from 37% to 57%. BCL2 and BCL6 gene rearrangements varied from 13% to 43% and from 30% to 44%,3-6,14,15 respectively. Next, FLs without t(14;18) and with/without BCL6 gene rearrangement were described as a unique FL subtype by some groups,5,16,17 but they showed the variability in morphologic and immunophenotypic findings (grade 3: 13%-78%; CD10+: 29%-36%). In this study, almost all (about 90%) CD10−MUM1+ FLs were high grade and were negative for BCL2 gene translocation and BCL6 gene abnormality (translocation or amplification). For these reasons, we consider CD10-MUM1+ FL to be related to previously described atypical FL, but we also suggest (on morphologic, phenotypic, and genetic grounds) that it forms a more homogeneous disease entity. Our findings may also carry the clinically interesting implication that a minority of DLBCLs localize anatomically to lymphoid follicles and thereby mimic classical FL, when in reality they are unrelated to the latter disorder. Several characteristics of our patients (eg, the frequencies of BCL2 and BCL6 gene rearrangement, the poor survival) are consistent with this hypothesis.
Authorship
Contribution: K.K. and K.O. designed the study; K.K., N.N., F.A., and Y.G. executed the study and analyzed data; K.K. wrote the paper; and J.S., Y.S., Y.N., K.Y., K.S., S.Y., H.K., M.T., M.K., O.T., H.T., and M.S. partly contributed to analysis of data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
K.K. and G.Y. equally contributed to this study.
Correspondence: Kennosuke Karube, Department of Pathology, School of Medicine, Kurume University, Asahimachi 67, Kurume 830-0011, Japan; e-mail: karube1975@yahoo.co.jp.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This study was supported in part by Grants-in-Aid from the Ministry of Education, Science and Culture, Japan. K.K. is a Japanese Society for the Promotion of Science (JSPS) Research Fellow.
The authors thank Dr Y. Nakamura, Ms E. Sato, and the staff of St Mary's Hospital for their advice on the FISH method, as well as Mrs. K. Takasu for her technical support.