Abstract
Despite the widespread adoption of reduced-intensity conditioning (RIC) for myeloma, there are few data comparing outcomes with RIC with myeloablative conditioning (MAC). We report the outcomes of patients undergoing allogeneic transplantations for myeloma and reported to the EBMT. A minimum data set was available on 320 RIC and 196 MAC allografts performed between 1998 and 2002. The RIC patients were older (51 vs 45 years) with more progressive disease (28% vs 21%) and more had received a prior transplant (76% vs 11%). In addition, there was a longer time to transplantation and an increased use of peripheral blood and T-cell depletion. For RIC and MAC, respectively, the nonrelapse mortality (NRM) at 2 years was 24% and 37% (P = .002); overall survival, 38.1% and 50.8% (not significant [ns]); and progression-free survival (PFS), 18.9% and 34.5% (P = .001). On multivariate analysis, RIC was associated with a reduction in NRM (HR, 0.5), but this was offset by an increase in relapse risk (HR, 2.0), and the conditioning intensity did not impact on overall survival or retain significance for PFS. These data suggest that there is a continuing need to investigate dose intensity in the conditioning for myeloma allografts.
Introduction
Myeloma is currently the most frequent indication for hematopoietic stem cell transplantation within Europe and North America.1,2 The majority of transplantations are autologous and as such represent the most effective palliation for these patients. Even the double autologous transplantation approaches where there is demonstrable good long-term control in a minority of patients do not appear to result in cure of the disease.3 Allogeneic transplantation is associated with a lower incidence of disease relapse, however initial enthusiasm in the 1990s waned because of the high transplantation-related mortality reported in many series.4,5 A previous registry analysis from the European Group for Blood and Marrow Transplantation (EBMT) compared autologous and sibling allogeneic transplantation. Autologous transplantation was associated with a lower mortality and improved overall survival. However, patients undergoing allogeneic transplantation had a lower relapse risk, and those allograft patients surviving more than one year after transplantation had a survival advantage.6 The advent of reduced-intensity conditioning (RIC) regimens has resulted in renewed interest in allogeneic transplantation as a treatment modality for myeloma. The pioneering studies led by groups in Seattle, Jerusalem, and Houston all suggest that ablation of host hematopoiesis can be successfully achieved by immunologic means rather than with cytotoxic chemotherapy or radiotherapy.7-10 This is associated with a reduction in nonrelapse mortality (NRM) and has led to a reassessment of age-related criteria for allogeneic transplantation.
Initial studies of RIC in myeloma patients were encouraging with NRMs of 2% to 11% and overall and disease-free survivals of 60% to 70%.11,12 With further follow-up and in other studies, NRM rates have been higher (17%-28%), and relapse has become an increasingly apparent problem with progression-free survivals (PFSs) in the region of 30% to 36%.12,13 This has been confirmed in a recent registry-based analysis of 229 patients who underwent transplantation with a variety of RIC regimens from 33 centers across Europe that suggested outcomes are less impressive and notably did not provide any evidence of durable disease-free survival.14 Moreover, results with conventional myeloablative conditioning are not universally disappointing, and NRMs of 22% have been reported using melphalan and total body irradiation (TBI).15 Some of these differences may be explained by improvements in supportive care, and this is consistent with the observation of an improvement in survival with time after allogeneic transplantation.16 Against this background, there has been a near-complete switch from conventional to reduced-intensity conditioning regimens between 1998 and 2003. This study compares the outcomes of patients receiving allografts with conventional myeloablative conditioning (MAC) with patients receiving allografts with RIC.
Patients, materials, and methods
This study was conducted on behalf of the Chronic Leukaemia Working Party (CLWP) of the EBMT. The EBMT represents more than 500 transplantation centers in and beyond Europe. All EBMT centers report a minimum essential data set into a central database for all transplantations conducted. Many centers also report a more comprehensive data set and more than 2500 allogeneic transplantations have been reported to the myeloma registry. Informed consent was obtained locally according to the regulations applicable at the time of transplantation. Since January 1, 2003, the EBMT has required centers to confirm that written informed consent has been obtained prior to data acceptance. Data on consent are incomplete; however, all patients on whom there are data have consented for data submission to the registry. The minimum data set required for inclusion of a patient in the study was defined at the outset and included the following: diagnosis, disease status at transplantation, donor type, conditioning regimen, number of prior transplantations, grade of acute graft-versus-host disease (aGvHD), remission status after transplantation, disease status at follow-up, overall survival, disease-free survival, NRM, and relapse. The analysis was restricted to first allogeneic transplantations only; in addition, because of a change in practice with year of transplantation, the analysis was restricted to those performed between January 1, 1998, to December 31, 2002. The following parameters were available for the majority of patients and were entered into the analysis: disease stage at diagnosis (477 [92.3%] of 516 patients), isotype of myeloma (513 [99.4%] of 516), donor sex (494 [95.7%] of 516), nature of GvHD prophylaxis (421 [81.6%] of 516), and time to neutrophil engraftment (457 [88.6%] of 516). Data on patient cytomegalovirus (CMV) status were only available on 85.9% of patients (443 of 516).
Definitions
The diagnosis of myeloma was based on local review. In the absence of a universally agreed definition of RIC, the local center's definition of RIC was used provided the conditioning regimen fell within these criteria: a melphalan dose of 140 mg/m2 or less, a busulfan dose of 8 mg/kg or less, or a cyclophosphamide dose of less than 120 mg/kg. If TBI was used, a dose of radiation less than 6 Gy, or 6 Gy if fractionated, was accepted as RIC. Remission status was stratified into patients in first remission either partial (PR) or complete (CR); patients in second remission or patients failing to achieve a partial remission after induction chemotherapy (minimal response or stable disease); and patients undergoing transplantation with evidence of disease progression. Patients were considered to have had a tandem autograft–RIC allograft if the allograft had been performed within 6 months of the first transplantation, and/or if there was the stated intention of a planned tandem procedure provided there had been no relapse of the disease in the intertransplantation interval. T-cell depletion was defined as the use of ex vivo graft manipulation and/or the use of T-depleting antibodies. In the analyses, the use of alemtuzumab was separated from other forms of T-cell depletion. Response, relapse, and disease progression were defined according to EBMT criteria, published by Blade et al.17 Response was documented on or after day 100 after transplantation. Survival was measured in months and defined as the time from the date of transplantation until date of death or last follow-up. Progression-free survival was defined as the time from transplantation until date of progression/death from any cause or last follow-up. NRM was defined as death due to any cause, which occurred without previous disease progression or relapse after transplantation.
Statistics
Comparisons between groups were made using the Chi-squared test for categoric data and the Mann-Whitney test for continuous data. Probabilities of overall survival (OS) and PFS were calculated using the Kaplan-Meier method, while probabilities of NRM and relapse were estimated using the cumulative incidence nonparametric estimator. All time-to-event outcomes were truncated at 36 months due to differential follow-up of the MAC and RIC groups. To assess the role of acute GvHD on PFS and OS, a landmark analysis was applied on patients who were failure free at 100 days. Univariate comparisons were made using the log-rank test for OS and PFS and the Gray test for NRM and relapse incidence, and variables found to be significant at the P < .1 level were entered into a proportional hazards regression analysis using a backward stepping procedure. Multivariate analysis was made using the Cox proportional hazards model. The following factors were considered in the univariate and multivariate analyses: conditioning intensity, patient sex, age, disease stage, immunoglobulin isotype, number of lines of prior therapy, disease status at conditioning, year of transplantation, interval from diagnosis to transplantation, use of T-cell depletion and antibody therapy, source of stem cells, donor type, donor sex and donor-recipient sex combinations, and graft-versus-host prophylaxis. Beta 2 microglobulin, CMV, and donor age were excluded due to incomplete data. All quoted P values are from 2-sided tests. Quoted confidence intervals (CIs) refer to 95% boundaries.
Results
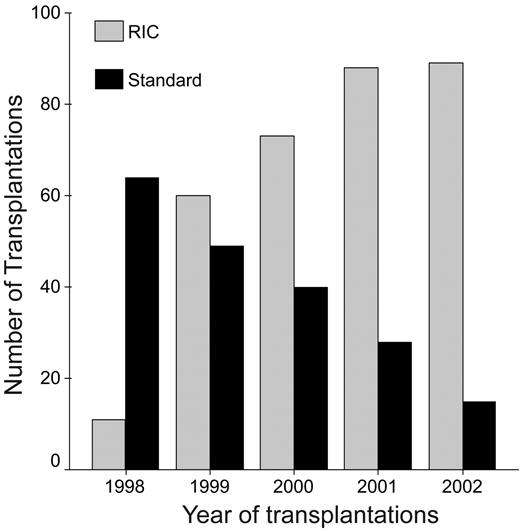
Data were available on a total of 516 patients from 103 centers: 320 patients with RIC and 196 with MAC. The median follow-up was 28 months; however, it was longer for the MAC patients than the RIC patients (median, 36 vs 23 months; P < .001). The patient characteristics are shown in Table 1. There were significant differences between the groups, with RIC patients being older (median age, 51 vs 45 years) and with more progressive disease (PD; 28% vs 21%). The majority of RIC patients had had a prior autologous transplantation, and for many of these it was as part of planned double transplantation procedure. Unsurprisingly, there were also a number of differences in the transplantation-related characteristics (Table 2). A number of these differences are due to the greater focus on immunosuppression and higher stem cell doses used in the RIC compared with the MAC regimens. This is reflected both in the increased use of peripheral blood stem cells (PBSCs) and the increased use of T-depleting antibodies such as alemtuzumab (Campath IH) or antithymocyte globulin (ATG) in the RIC group. Likewise, GvHD prophylaxis other than the conventional cyclosporine and methotrexate was also used more frequently in the RIC patients. The other major differences were increased time to transplantation (median, 18 vs 9 months) and increased use of unrelated donors (15% vs 8%). Finally, there was an almost complete change in practice, with a progressive switch from MAC to RIC over the 5 years of this study (Figure 1).
Use of reduced-intensity and myeloablative conditioning with respect to year of transplantation.
Use of reduced-intensity and myeloablative conditioning with respect to year of transplantation.
Conditioning regimens
A variety of different conditioning regimens was used, however the common regimens for the MAC patients were melphalan and TBI (n = 72; 36.7%) or cyclophosphamide and TBI (n = 96; 49.0%). Only 30 patients (15.3%) did not receive a TBI-based regimen. A total of 18 different RIC regimens were used, however the combinations of fludarabine with melphalan, busulfan, or low-dose TBI represented 86% of regimens (Table 3). Where TBI was used, the median dose was 12 Gy in a median of 3 fractions in the MAC group (range, 8-14.4 Gy in 1-8 fractions) and 2.0 Gy in a median of 1 fraction (range, 1.2-6.0 Gy in 1-6 fractions) in the RIC group. In those RIC patients receiving fludarabine and TBI, the median dose was 2.0 Gy (range, 1.8-2.0 Gy; data missing in 11 cases). T-cell depletion was used more frequently in the RIC rather than the MAC patients (60% vs 48.5%; P = .002). In the RIC patients, this was achieved most frequently with either ATG or alemtuzumab. The majority of RIC patients receiving alemtuzumab received it in combination with fludarabine and melphalan (58/65). Ex vivo methods of T-cell depletion were used more frequently in the MAC compared with the RIC patients (37.2% vs 2.5%; P < .001).
Engraftment and graft failure
Data on engraftment were available in 98.3% of patients, and in 87% of patients data on the time to achieve a neutrophil count more than 0.5 × 109/L were available. Eight RIC patients failed to engraft and 1 subsequently reverted to host chimerism (secondary graft failure, 2.8%), whereas 11 (5.6%, not significant [ns]) of the MAC patients failed to engraft with no secondary graft failures. The median time to neutrophil recovery was 15.0 days, with no difference between the RIC and the MAC patients (P = .13). The use of peripheral blood rather than bone marrow was associated with a shorter time to neutrophil engraftment in patients with MAC (median, 15 vs 20 days; P < .001), but was no different for patients with RIC. The use of methotrexate as GvHD prophylaxis was also associated with delayed neutrophil engraftment (median, 16 vs 14 days; P = .004).
Graft-versus-host disease
Overall, 294 patients (56.9%) developed some aGvHD, with 26.3% developing grade II and 13.1% grades III to IV. The incidence was significantly lower in the RIC patients, with 114 patients (35.5%) compared with 90 patients (45.9%) developing grades II to IV aGVHD (P < .02). The use of an unrelated donor was associated with the development of more grades II to IV aGVHD (52.3% vs 36.9%; P = .007). No relationship between GvHD prophylaxis, the use of T-cell depletion, the source of stem cells (peripheral blood vs bone marrow), the donor-recipient sex combinations, era of transplantation, and the development or severity of aGvHD could be demonstrated. Chronic GvHD (cGvHD) was evaluable in 329 of 448 patients surviving beyond day 100. Of survivors, 163 (49.5%) developed some cGvHD, with 27.1% (89 patients) and 22.5% (74 patients) of the evaluable patients developing limited and extensive cGvHD, respectively. There was no difference between conditioning regimen and the development or severity of cGvHD. The use of T-cell depletion was associated with a reduction in cGvHD (53.7% vs 77%; P < .001), whereas male recipients with female donors and the use of PBSCs were both associated with a higher incidence (60% vs 46%; P < .001) and (51.5% vs 44.7%; P = .03), respectively. Graft-versus-host disease is an important factor determining survival. The development of aGvHD was associated with an adverse impact on NRM, which was 32.5% for patients with grades II to IV aGvHD compared with 14.8% in those with grades 0 to I (P < .001). This resulted in an adverse impact: overall survival beyond 100 days was 43% and 56% at 3 years for grades II to IV and 0 to I aGvHD, respectively (P < .001).
Nonrelapse mortality
The overall NRM was 10.5% at 100 days but rose to 29% (CI, 32%-41%) at 2 years. The NRM at 2 years was 24% (CI, 19%-29%) in patients who underwent transplantation with RIC and was significantly lower compared with patients who underwent transplantation with MAC (37%; CI, 31%-45%; P = .002; Figure 2); and this retained significance in the multivariate models (hazard ratio [HR], 1.9; CI, 1.2-2.8; P = .003). The other factors associated with an increased NRM were remission status at transplantation, with an HR of 1.9 in patients with advanced disease at the time of transplantation (Table 4). A prior autologous transplantation was of borderline significance (HR, 1.6; CI, 1.0-2.5); however, if it was performed as a planned tandem allograft or an allograft within 6 months of an autograft without interval relapse, the NRM was the same as for patients undergoing a first transplantation.
Nonrelapse mortality in 516 patients with myeloma who underwent transplantation with reduced-intensity or myeloablative conditioning.
Nonrelapse mortality in 516 patients with myeloma who underwent transplantation with reduced-intensity or myeloablative conditioning.
Disease response, progression-free survival, and disease-free survival
Four hundred and fifty five patients were evaluable for response. The overall response rate was 84.2%. The response rate was higher in patients with MAC (CR, 53.4%; PR, 38.0%) than the RIC patients (CR, 33.6; PR, 46.6; P < .001). Chemosensitive disease at the time of conditioning was associated with a better posttransplantation response, 43.4% CR and 42.9% PR compared with 22.0% and 47.5%, respectively (P = .001). The use of T-cell depletion was associated with a lower CR rate (37.9% vs 46.5%, P = .04); the complete response rate was lower still with the use of alemtuzumab (CR, 28.1%; P = .01). Of the 185 patients who achieved a CR, 51 patients have relapsed, and of the remaining 332 patients, 131 have progressed, giving a cumulative incidence of relapse or progression at 3 years of 42.3% (CI, 37.5%-47.8%).
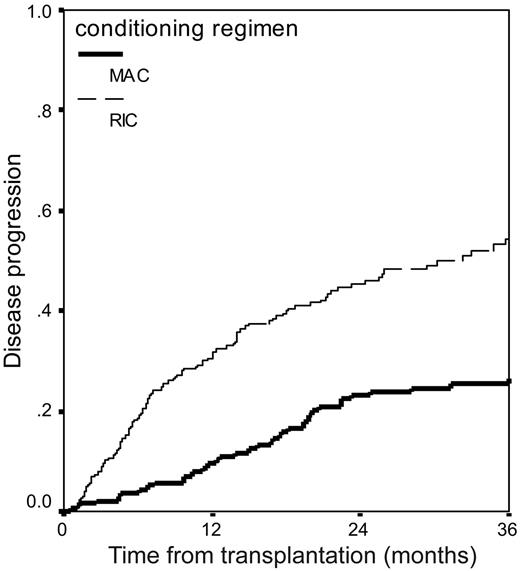
The use of any T-cell depletion was also associated with an increased risk of disease relapse or progression with this effect being most marked for patients receiving alemtuzumab (HR, 2.0; CI, 1.4-2.9; P < .001). The use of RIC was also associated with twice the relapse risk of the use of MAC: 54% (CI, 48%-62%) versus 27% (CI, 20%-34%) (Table 4; Figure 3). Disease remission status at transplantation was also an important factor. The relapse risk was also not stable over this 5-year period, with a lower risk of relapse being seen in 2000 to 2002 (Table 4). This was apparent despite the switch away from MAC to RIC that occurred during the same period.
Cumulative incidence of disease relapse or progression in 516 patients with myeloma who underwent transplantation with reduced-intensity or myeloablative conditioning.
Cumulative incidence of disease relapse or progression in 516 patients with myeloma who underwent transplantation with reduced-intensity or myeloablative conditioning.
The overall PFS was 26.3% at 36 months, with no evidence of a plateau to the curve. The use of RIC was associated with inferior PFS: 18.9% versus 34.5% at 3 years (P = .001). The conditioning type did not, however, retain significance on multivariate analysis. The factors that were predictive were advanced disease at transplantation (HR up to 2.36; CI, 1.7-3.28; P < .001) and the use of alemtuzumab in the conditioning (HR, 1.9; CI, 1.48-2.53; P < .001; Table 4).
Survival
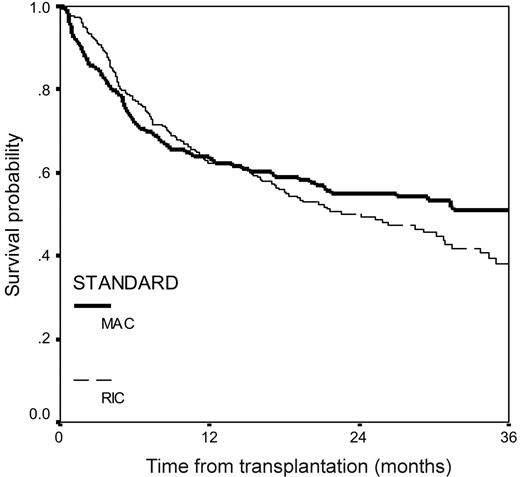
The median survival from transplantation for the whole group was 29 months (CI, 22-36 months). For the MAC patients, the projected 3-year survival was 50.8% and was not significantly different from RIC patients at 38.1% (Figure 4). The factors that predicted overall survival on multivariate analysis were progressive disease at the time of conditioning (HR, 2.4; CI, 1.0-3.4; P < .001) and the use of alemtuzumab in the conditioning (HR, 1.8; CI, 1.1-2.0; P < .001; Table 4).
Overall survival from transplantation in 516 patients with myeloma who underwent transplantation with reduced-intensity or myeloablative conditioning.
Overall survival from transplantation in 516 patients with myeloma who underwent transplantation with reduced-intensity or myeloablative conditioning.
RIC versus MAC
The factors associated with the main outcome variables of survival, PFS, and NRM are different for the RIC compared with the MAC strategies. In a multivariate analysis limited to the RIC patients only, progressive disease at transplantation is associated with an adverse OS, PFS, NRM, and relapse risk (HR, 2.0, 1.9, 1.9, and 1.9, respectively; Table 5). Overall survival, PFS, and relapse were also inferior in patients receiving alemtuzumab (HR, 1.8, 1.8, and 2.3, respectively), and the use of any form of T-cell depletion corresponded to a higher relapse rate (HR, 1.6). For patients receiving MAC, the use of alemtuzumab was significant with respect to relapse risk only. Disease status was less important, however the use of a nonsibling donor correlated with an inferior OS, PFS, and NRM (HR, 2.0, 2.0, and 1.7, respectively; Table 6). A prior autologous transplantation was also a significant risk factor after MAC with the HR for OS, PFS, and relapse being 1.8, 2.1, and 3.5, respectively, but no association between autologous transplantation and NRM could be demonstrated. The effect of different conditioning regimens cannot be separated from conditioning intensity. For the RIC patients, no difference in overall survival or PFS was seen between patients conditioned with fludarabine and TBI, fludarabine and melphalan, and fludarabine and busulfan combinations. For the MAC patients, the use of cyclophosphamide and TBI was associated with a slightly better overall survival (54.8% vs 47.5% at 3 years; P = .04). However, this did not retain significance on multivariate analysis.
Discussion
Allogeneic hematopoietic stem cell transplantation is recognized to be an effective strategy in what is otherwise an incurable disease with a relatively short median survival. Results with conventional conditioning regimens in the 1990s were, however, disappointing due to NRM. RIC offers the prospect of retaining the allogeneic graft-versus-myeloma (GvM) effects of transplantation while reducing regimen-related toxicity and mortality. This study confirms that there has been a widespread move away from MAC to the use of RIC for myeloma allografts. This change has occurred in the absence of evidence supporting the efficacy of RIC. Randomized studies comparing RIC with MAC, which might definitively answer this question, are not available and are not likely to become available in the immediate future. The use of registry data to address these questions is therefore reasonable but is associated with potential problems. There are many differences among patients receiving RIC regimens and those receiving MAC regimens. Many of these differences such as age and comorbidities are directly linked to the physician's decision on conditioning intensity. Other factors such as the type of chemotherapy and the use of T-cell–depleting antibodies, GvHD prophylaxis, and cell source are linked to the nature of the conditioning regimen and make case-matched methodology problematic. Examples of this are cell source and the use of prior autologous transplantation. In the majority of MAC patients, a prior autograft represented a previously failed therapy and is associated with an adverse outcome. However, in many RIC patients, an autologous transplantation was a planned cytoreductive strategy before the allograft. The physician's treatment strategy was unknown in many patients. We have made the assumption that patients receiving an allogeneic transplant within 6 months of an autologous transplant without evidence of interval disease relapse were effectively having an auto-RIC allograft strategy and included them in the “tandem” transplantation group. Prior transplantation is likely to be associated with a higher risk for NRM, and this was of borderline significance on the multivariate model in this study. The tandem group had an NRM that was not significantly different from those patients receiving a first transplant. This would support the tandem strategy in RIC, however it is important to note that this is not an intention-to-treat analysis, as the number of patients in whom a tandem autograft–RIC allograft strategy was planned but did not go on to receive the second transplant is unknown.
The use of T-cell depletion was an important factor influencing relapse, PFS, and OS, and although its use was significantly higher in RIC regimens it remained important in MAC. T-cell depletion methodologies and doses of T-depleting antibodies differ considerably and were either not available or the groups were too small to permit detailed subanalysis. Analysis of the use of ALG, alemtuzumab, or ex vivo graft manipulation suggested that the effect was most pronounced for the use of alemtuzumab, and it was the use of this drug rather than T-cell depletion as a whole that retained greatest significance in the multivariate models. This would suggest that caution needs to be exercised in the use of T-cell depletion and alemtuzumab in particular in myeloma allografts. The close linkage between RIC and T-cell depletion means that although RIC was not associated with an adverse PFS in this study, it may become relevant in a larger series or if a series could be identified that could be matched for T-cell depletion.
The NRM in the MAC patients remains significant at 25% at 6 months and 37% at 2 years. This is consistent with outcome data reported by the EBMT for patients who underwent transplantation between 1994 to 199816 ; however it does not provide evidence of a continued improvement with time. The NRM is significantly lower in the RIC patients than the MAC patients; this is consistent with the majority of reports for the outcome of RIC transplantations both in myeloma and in other hematologic malignancies.18 The RIC patients in general underwent transplantation in the latter period of this study, and it is possible that the reduction in NRM may reflect other improvements in supportive care. Improvements in transplantation outcome related in part to improvements in supportive care and management of infections in particular are well documented.19 This study, however, was restricted to a 5-year time window, and changes in supportive care over this period are likely to be more modest. Given the lower doses of chemotherapy and radiotherapy, a reduction in conditioning-related morbidity and mortality is expected. A reduction in aGvHD has also been variably reported and may be due to changes in the cytokine profile during the immediate posttransplantation period and related to the lower nonhematologic toxicity of the RIC regimens. The time of onset of aGvHD had also been reported to be delayed after RIC allografts, and aGVHD starting after day 100 is reported.20 It is not possible to exclude the possibility that some of the cGvHD may represent late-onset aGvHD.
Patient selection may also play a role, however this is likely to reduce rather than increase any benefit of RIC as more elderly patients, more patients with chemoresistant disease, and those with a longer interval from diagnosis to transplantation received RIC conditioning.
Any advantage of RIC in reducing NRM, however, is offset by an increased relapse risk. This would suggest either that the allogeneic effect is less potent in the setting of RIC conditioning or more likely that there remains benefit from high-dose chemotherapy and the allogeneic GvM effect does not substitute entirely. The GvM effect cannot, however, be dismissed and remains significant, as evident by the increased relapse risk with T-cell depletion in general and with alemtuzumab in particular. Whether the GvM effect can be clearly separated from a less specific alloimmune effect remains uncertain.
While relapse is clearly important and undesirable, it does not necessarily reflect a complete failure of the transplantation strategy. Salvage of patients with further chemotherapy or donor lymphocyte infusion (DLI)21-23 may provide useful and potentially durable disease control; as such, relapse is not an end point that carries the same weight as NRM or OS. Unfortunately, there were insufficient data available in this cohort on outcome after relapse to construct current myeloma-free survival curves, which would have been of interest. T-cell depletion is, however, an important factor in relapse and survival and appears to outweigh the influence of conditioning regimen, per se; however, given the link between RIC and T-cell depletion it is not possible to be entirely confident that the use of alemtuzumab is associated with an inferior PFS but that RIC is not.
The major limitations of this study are the intrinsic differences between the population of patients receiving RIC and MAC regimens. We have used multivariate models to try to disentangle the patient differences, however there is a limit to the ability of these models to resolve some of the associations that are closely linked with type of conditioning and for this reason we have avoided subgroup analyses. There is considerable heterogeneity within the RIC regimens in particular; it is also possible that we may have “overlooked” specific regimens or strategies that may represent an ideal balance of the advantages of the MAC and RIC groups, and it will require randomized or quasirandomized prospective studies to elucidate these.
Despite these caveats, these data do not support the universal use of RIC for myeloma allografts and suggest the following: (1) attention needs to be paid to RIC strategies including conditioning, the use of prior autograft, immunosuppression, and the role of donor lymphocyte infusions; (2) there may still be a role for conventional MAC regimens in a selected population of patients with myeloma.
Authorship
Contribution: G.G. and C.C. proposed the study; S.I. and C.C. analyzed the data; G.G., D.N., B.B., and J.F.A. reviewed the data and provided valuable critique of the study; and C.C. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the members of the Chronic Leukaemia Working Party of the EBMT is included as Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Charles Crawley, Department of Haematology, Box 234, Addenbrooke's Hospital, Hills Rd, Cambridge, CB2 0QQ; e-mail: charles.crawley@addenbrookes.nhs.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Anja van Biesen and the registry office in Leiden, The Netherlands.