Abstract
We investigated if an infusion of alloreactive natural killer (NK) cells would reduce GVHD and mediate antitumor effects in mice undergoing MHC-matched allogeneic stem cell transplantation (SCT). Balb/c mice bearing RENCA tumors underwent an allogeneic SCT from MHC-matched B10.d2 donors and were given a single infusion of either Ly49 ligand-matched, ligand-mismatched, or no donor NK cells. Recipients of Ly49 ligand-mismatched NK cells had a reduced incidence of graft-versus-host disease (GVHD; 39% vs 100%; P < .01), and prolonged survival (median 84 days vs 39 days; P < .01) compared with SCT recipients not receiving NK cells. Recipients of Ly49 ligand-matched NK cells had the same incidence of GVHD and similar survival compared with controls not receiving NK cells. Pulmonary tumor burden was significantly (P < .01) lower in recipients that received Ly49-mismatched or Ly49-matched NK cells compared with recipients not receiving NK cells. These data provide in vivo evidence that a single infusion of alloreactive donor NK cells reduces GVHD and mediates antitumor effects following MHC-matched allogeneic transplantation.
Introduction
Killer immunoglobulin-like receptors (KIRs) and the leukocyte immunoglobulin-like receptor-1 (LIR-1) may inhibit or activate natural killer (NK) cells.1 While most human KIRs are specific for MHC class I molecules, mice have evolved convergent receptors of the C-type lectin superfamily, termed Ly49. Analogous to human NK KIRs, subgroups of MHC-specific Ly49 receptors are expressed on murine NK cells and regulate their function.2
In MHC-mismatched transplantation, the infusion of alloreactive Ly49-mismatched NK cells (defined as an NK cell population expressing a Ly49 receptor for which the recipient lacks a corresponding inhibitory MHC class I ligand) decreases the incidence of graft-versus-host disease (GVHD).3 In humans, T-cell–depleted allogeneic stem cell transplantations (SCTs) have a similar reduction in GVHD and leukemia relapse when KIR ligand incompatibility exists.3,4 Although GVHD-associated morbidity represents a major obstacle in allogeneic SCT, it is closely associated with beneficial graft-versus-tumor (GVT) effects.5 T-cell depletion reduces GVHD but increases the risk of infectious complications and disease relapse.6,7
It is unknown if alloreactive NK cells can reduce GVHD and simultaneously induce antitumor effects in MHC-matched T-cell–replete SCT. We studied the effect of adoptively infusing Ly49 ligand-mismatched NK cells on GVHD and GVT in an MHC-matched T-cell–replete murine model of allogeneic SCT.
Materials and methods
Animals
Balb/C (H-2d), B10.d2 (H-2d), C57BL/6 (H-2b), and CB6F1 (H-2d/b) (Jackson Laboratory, Bar Harbor, ME) mice (8-15 weeks old) were housed in a pathogen-free facility in microisolator cages and received acidified water containing neomycin. All experiments were approved by the National Heart, Lung, and Blood Institute (NHLBI) animal care and use committee (protocol H-0112).
Preparation of NK cells
NK cells enriched from splenocytes by magnetic bead negative depletion (Miltenyi Biotec, Auburn, CA) were labeled with Ly49G2, Ly49C, and Ly49D (BD Pharmingen, San Diego, CA) and flow sorted (MoFlo; DakoCytomation Carpinteria, CA) into 3 distinct NK cell populations: Ly49G2+/Ly49C−, Ly49C+/Ly49G2−, and Ly49G2+/Ly49D−. Sorted NK cells were cultured for 4 to 6 days in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 500 U/mL recombinant human interleukin-2 (Roche, Nutley, NJ).
Cytotoxicity assay
Chromium-51 assay was performed as previously described.8 Supernatants were harvested onto Luma plates (Perkin Elmer, Wellesley, MA) and analyzed using a MicroBeta scintillation counter (Perkin Elmer). NK cells were tested for cytotoxicity against Concanavalin A (Con-A) blasts, RENCA (H-2d: murine renal cell carcinoma; Dr Wiltrout, National Cancer Institute, National Institutes of Health) and B16-F10 (H-2b: murine melanoma) tumor cells (ATCC, Manassas, VA). Con-A blasts were prepared by culturing 5 × 106 splenocytes/mL in DMEM, 10% FCS, and 5 μg/mL Con-A (Sigma Aldrich, St Louis, MO) for 48 to 96 hours.
Transplantation model
Following 950 cGy total body irradiation, Balb/C transplant recipients were injected intravenously via the tail vein with 10 × 106 to 15 × 106 splenocytes and 8 × 106 bone marrow cells from B10.d2 donor mice. At 4 to 6 days after transplantation, recipients were injected intravenously with 0.4 × 106 to 1.0 × 106 purified NK cell subpopulations. In this model, NK cells expressing Ly49G2 (binds to H-2Dd) and Ly49C (binds to H-2Kb) represent ligand-matched “nonalloreactive” and ligand-mismatched “alloreactive” NK cells, respectively. Animals were monitored and graded for GVHD according to the following symptoms: alopecia (0-4 points); weight loss (0-2 points; < 5% = 0, 5%-15% = 1, > 15% = 2); hunched posture (0-2 points); and ear or eye irritation (0-1 point). To evaluate for antitumor effects, Balb/C recipients were injected intravenously with 1 × 105 RENCA tumor cells (Balb/C origin) 10 days before transplantation. Lung weights and organ tumor infiltration were evaluated after death or at the termination of the experiment (100 days after SCT). Moribund animals were humanely killed according to NHLBI animal care guidelines.
Statistics
A Student t test or Fisher exact test was used to assess differences between groups. A P value of less than .05 was considered to be significant.
Results and discussion
In an MHC-matched model of allogeneic SCT, GVT effects occur when Balb/C (H-2d) recipients with RENCA tumors receive transplants with B10.d2 (H-2d) marrow and splenocytes. In this model, survival is longer in allogeneic SCT recipients compared with syngeneic SCT controls (mean 51 vs 31 days; P < .01), although death from lethal GVHD and/or tumor progression ultimately occurs (Figure 1). Therefore, we investigated if an infusion of allogeneic Ly49 ligand-mismatched alloreactive NK cells could protect tumor-bearing recipients of an MHC-matched SCT from GVHD and deliver an effective antitumor response. Ly49 ligand-matched (Ly49G2) and ligand-mismatched (Ly49C) NK cells for H-2d recipients were isolated and tested in vitro for cytotoxicity against Con-A blasts of Balb/C and C57BL/6 origin. As predicted,9-11 Ly49C NK cells demonstrated increased cytotoxicity against Balb/C Con-A blasts compared with Con-A blasts of C57BL/6 origin, whereas Ly49G2 NK cells were more cytotoxic against C57BL/6 Con-A blasts (Figure 1). In vitro, allogeneic NK cells from B10.d2, BL/6, and CB6F1 mice had enhanced cytotoxicity against Balb/C RENCA tumor cells compared with syngeneic Balb/C NK cells. However, in contrast to Balb/C Con-A blasts, high surface expression of H-2Dd on RENCA cells (data not shown) did not protect from lysis by allogeneic Ly49G2 NK cells; in vitro, Ly49G2 and Ly49C ligand-mismatched NK cells exhibited comparable cytotoxicity against RENCA (Figure 1). These findings are consistent with a prior report by Sentman et al11 showing that MHC/Ly49-matching protects lymphoblasts but not tumor cell lines from NK lysis. It is unclear why Ly49G2+ NK cell cytotoxicity was not inhibited by RENCA MHC molecules. Under certain conditions, H-2Dd can ligate the activating receptor Ly49D, overriding other pathways that inhibit NK cell function.12 However, Ly49G2+/Ly49D− purified NK cells maintained high levels of in vitro cytotoxicity against RENCA, making it unlikely that NK cell activation through Ly49D overrode Ly49G2-mediated inhibition (Figure 1). For perforin-mediated lysis of RENCA, NK cell activation through NKG2D by ligands expressed on target cells has been shown to be crucial.13 Activation through NKG2D in some cases overrides inhibitory signaling mediated by MHC class I molecules.14 Variability in expression of NKG2D ligands or differences in NKG2D expression on NK cell subsets could potentially account for these findings. It is also possible that tumor-specific proteins may alter the Ly49 binding site of H-2Dd, preventing RENCA MHC molecules from inactivating NK cells through the Ly49 receptor.11
Survival in murine recipients with RENCA tumors undergoing syngeneic versus MHC-matched transplantation and in vitro cytotoxicity of NK cell subpopulations against Balb/C and C57BL/6 Con-A blasts and tumor cells. Survival of Balb/C mice challenged with RENCA tumor cells that underwent SCT with allogeneic B10.d2 (⋄) or syngeneic Balb/C (♦) splenocytes (15 × 106) and bone marrow cells (8 × 106) (top panel). Images show a representative picture of lung metastases in an allogeneic (day 53) and syngeneic (day 31) transplant recipient. Arrows show RENCA tumor foci in an allogeneic transplant recipient. Lungs in syngeneic recipients were replaced by tumor nodules. Significantly higher lung weights from increased tumor burden were observed at death in recipients of syngeneic (0.70 g ± 0.07 g) vs allogeneic (0.51 g ± 0.05 g; P < .01) transplants (A). NK cells isolated from CB6F1 mice by negative depletion magnetic beads (> 95% DX5+/CD3−) were flow sorted into Ly49G2+ and Ly49C+ subpopulations, then cultured for 4 days in IL-2–containing media and stained for purity by flow cytometry (B) and tested for lytic activity against Con-A blasts and RENCA (H-2d) and B16 (H-2b) tumor cells of Balb/C (H-2d) and C57BL/6 (H-2b) origin, respectively. Ly49C+/Ly49G2− (○), Ly49G2+/Ly49C− (•), and Ly49G2+/Ly49C−/Ly49D− (▴) (C). NK cell subpopulations isolated from various strains of mice tested against Balb/C Con-A blasts and RENCA tumor cells (effector-target ratio [E/T] = 4:1) (D). Error bars depict SD of mean. One of between 3 and 5 representative experiments is shown. *P < .05 compared with opposing Ly49 NK group; unpaired t test. **The difference in survival between recipients of syngeneic and allogeneic transplantation is significant; P < .01, unpaired t test.
Survival in murine recipients with RENCA tumors undergoing syngeneic versus MHC-matched transplantation and in vitro cytotoxicity of NK cell subpopulations against Balb/C and C57BL/6 Con-A blasts and tumor cells. Survival of Balb/C mice challenged with RENCA tumor cells that underwent SCT with allogeneic B10.d2 (⋄) or syngeneic Balb/C (♦) splenocytes (15 × 106) and bone marrow cells (8 × 106) (top panel). Images show a representative picture of lung metastases in an allogeneic (day 53) and syngeneic (day 31) transplant recipient. Arrows show RENCA tumor foci in an allogeneic transplant recipient. Lungs in syngeneic recipients were replaced by tumor nodules. Significantly higher lung weights from increased tumor burden were observed at death in recipients of syngeneic (0.70 g ± 0.07 g) vs allogeneic (0.51 g ± 0.05 g; P < .01) transplants (A). NK cells isolated from CB6F1 mice by negative depletion magnetic beads (> 95% DX5+/CD3−) were flow sorted into Ly49G2+ and Ly49C+ subpopulations, then cultured for 4 days in IL-2–containing media and stained for purity by flow cytometry (B) and tested for lytic activity against Con-A blasts and RENCA (H-2d) and B16 (H-2b) tumor cells of Balb/C (H-2d) and C57BL/6 (H-2b) origin, respectively. Ly49C+/Ly49G2− (○), Ly49G2+/Ly49C− (•), and Ly49G2+/Ly49C−/Ly49D− (▴) (C). NK cell subpopulations isolated from various strains of mice tested against Balb/C Con-A blasts and RENCA tumor cells (effector-target ratio [E/T] = 4:1) (D). Error bars depict SD of mean. One of between 3 and 5 representative experiments is shown. *P < .05 compared with opposing Ly49 NK group; unpaired t test. **The difference in survival between recipients of syngeneic and allogeneic transplantation is significant; P < .01, unpaired t test.
The administration of a single infusion of donor B10.d2 Ly49 ligand-mismatched NK cells significantly reduced the incidence of skin GVHD (P < .01) and lowered the cumulative GVHD score (P < .05) in Balb/C recipients compared with SCT recipients receiving Ly49 ligand-matched NK cells or no NK cells. Ly49 ligand-mismatched NK cells also appeared to mediate antitumor effects that prolonged survival in transplant recipients with established pulmonary metastases (Figure 2). Postmortem evaluation of mice surviving at least 50 days showed no evidence of tumor in 43% of mice receiving Ly49 ligand-mismatched NK cells compared with 17% (P = .56) that received Ly49 ligand-matched NK cells and 0% (P = .24) that did not receive NK cells. Although this difference did not reach statistical significance, pulmonary tumor burden (evaluated by lung weights) was significantly lower in SCT recipients that received either Ly49-mismatched (0.45 g ± 0.09 g) or Ly49-matched NK-cells (0.43 g ± 0.08 g) compared with mice not receiving NK cells (0.67 g ± 0.02 g; P < .01). A trend toward longer survival in recipients of Ly49G2 ligand-matched NK cells compared with non–NK cell controls was observed (median 51 days vs 39 days; P = .10), yet the incidence and severity of GVHD did not differ between these cohorts. These data suggest that allogeneic NK cells mediate antitumor effects in vivo even in the absence of Ly49 incompatibility. Both Ly49C and Ly49G2 purified NK cells from B10.d2 mice were significantly (P < .01) more cytotoxic in vitro against RENCA cells compared with their autologous NK cell counterparts of Balb/C origin (Figure 1). These data suggest that allogeneic NK cells may have greater antitumor activity than autologous NK cells through mechanisms independent of currently defined NK cell activating and inhibitory pathways.
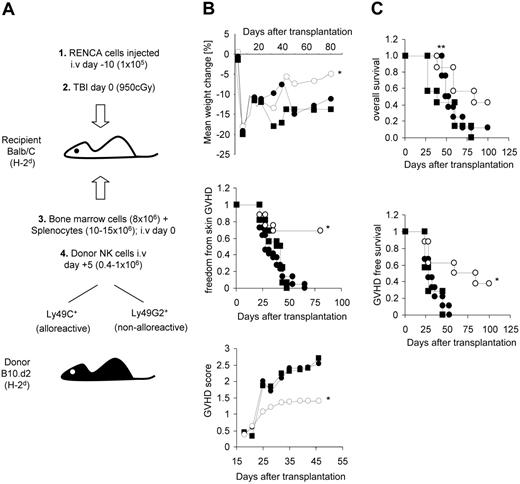
GVHD incidence and survival in tumor-bearing mice after allogeneic SCT given a single infusion of either Ly49 ligand-matched, ligand-mismatched, or no donor NK cells. Model of transplantation and adoptive NK cell infusion (A). NK cells expressing Ly49G2 (binds to H-2Dd) and Ly49C (binds to H-2Kb) represent ligand-matched “nonalloreactive” and ligand-mismatched “alloreactive” NK cells, respectively. Balb/C mice were irradiated (950 cGy) and underwent transplantation with 10 × 106 to 15 × 106 splenocytes and 8 × 106 bone marrow cells from B10.d2 donor mice. NK cells isolated from B10.d2 mice were expanded in 500 U/mL IL-2 for 5 days and injected 5 days after transplantation (0.4 × 106-1.0 × 106 NK cells/mouse) with Ly49G2+ NK cells (•), Ly49C+ NK cells (○), or no NK cells (▪). Recipients were monitored for development of symptoms of GVHD. Weight loss (top panel), freedom from skin GVHD (middle panel), and the mean cumulative GVHD score (bottom panel) were evaluated (B). Alopecia, hunched posture, loss of activity, and weight loss were noted before day 40 in the majority of mice that developed GVHD. Recipients injected with 105 RENCA tumor cells and 0.4 × 106 to 1.0 × 106 purified NK cell subpopulations on day 10 before transplantation and on day 5 after transplantation, respectively, were assessed for GVHD-free survival, overall survival, and tumor status by postmortem examination (C). Four pooled experiments with a total of 16 to 22 mice in each group are shown for panel B. Two pooled experiments with a total of 7 to 9 mice in each group are shown for panel C. *P < .05 compared with recipients of Ly49G2 NK cells and recipients of no NK cells; unpaired t test and Fisher exact test. **Recipients surviving longer than 50 days after transplantation were evaluated after death for the presence of lung metastases; 43% of mice receiving Ly49 ligand-mismatched NK cells, 17% of mice that received Ly49 ligand-matched NK cells, and 0% of mice that did not receive NK cells had no evidence of tumor.
GVHD incidence and survival in tumor-bearing mice after allogeneic SCT given a single infusion of either Ly49 ligand-matched, ligand-mismatched, or no donor NK cells. Model of transplantation and adoptive NK cell infusion (A). NK cells expressing Ly49G2 (binds to H-2Dd) and Ly49C (binds to H-2Kb) represent ligand-matched “nonalloreactive” and ligand-mismatched “alloreactive” NK cells, respectively. Balb/C mice were irradiated (950 cGy) and underwent transplantation with 10 × 106 to 15 × 106 splenocytes and 8 × 106 bone marrow cells from B10.d2 donor mice. NK cells isolated from B10.d2 mice were expanded in 500 U/mL IL-2 for 5 days and injected 5 days after transplantation (0.4 × 106-1.0 × 106 NK cells/mouse) with Ly49G2+ NK cells (•), Ly49C+ NK cells (○), or no NK cells (▪). Recipients were monitored for development of symptoms of GVHD. Weight loss (top panel), freedom from skin GVHD (middle panel), and the mean cumulative GVHD score (bottom panel) were evaluated (B). Alopecia, hunched posture, loss of activity, and weight loss were noted before day 40 in the majority of mice that developed GVHD. Recipients injected with 105 RENCA tumor cells and 0.4 × 106 to 1.0 × 106 purified NK cell subpopulations on day 10 before transplantation and on day 5 after transplantation, respectively, were assessed for GVHD-free survival, overall survival, and tumor status by postmortem examination (C). Four pooled experiments with a total of 16 to 22 mice in each group are shown for panel B. Two pooled experiments with a total of 7 to 9 mice in each group are shown for panel C. *P < .05 compared with recipients of Ly49G2 NK cells and recipients of no NK cells; unpaired t test and Fisher exact test. **Recipients surviving longer than 50 days after transplantation were evaluated after death for the presence of lung metastases; 43% of mice receiving Ly49 ligand-mismatched NK cells, 17% of mice that received Ly49 ligand-matched NK cells, and 0% of mice that did not receive NK cells had no evidence of tumor.
Asai et al15 previously demonstrated that allogeneic NK cell infusions prevented GVHD and delayed colon-cancer lung metastasis in MHC-mismatched transplant recipients. In this model, NK cell efficacy was linked to the timing of the NK cell infusion and was dependent on TGF-beta. By sorting NK cells into different subsets, we extend these observations, demonstrating that adoptively infused allogeneic Ly49-mismatched NK cells protect from GVHD and mediate antitumor effects in the setting of an MHC-matched SCT. Although both Ly49 subpopulations mediated antitumor effects against RENCA in vitro and in vivo, only Ly49-mismatched NK cells protected from GVHD and prolonged survival. Prior murine studies have shown that alloreactive NK cells can eradicate host antigen presenting cells (APCs). Both our in vitro and in vivo findings suggest that alloreactive Ly49-mismatched NK cells, when adoptively infused in the setting of an MHC-matched SCT, are cytotoxic to host APCs and RENCA tumor cells, resulting in a reduction in GVHD and the induction of antitumor effects, culminating in prolonged survival.
In humans undergoing T-cell–depleted HLA-mismatched transplantation with KIR incompatibility, the incidence of GVHD and acute myelogenous leukemia relapse is reduced. T-cell depletion delays T-cell recovery, which prevents GVHD but increases the risk of infectious complications.16 Recent reports suggest that T-cell–replete SCT with KIR ligand incompatibility may not afford the same beneficial effects of alloreactive NK cells on GVHD or disease relapse as observed with T-cell–depleted SCT.17 In our model, adoptively infused Ly49 ligand-mismatched NK cells were able to reduce GVHD and prolong survival using a transplant containing a large number of T cells that are vital to protect recipients from disease relapse and infectious complications. In humans, the genes encoding KIR and HLA molecules map to different chromosomes. A recent analysis of HLA and KIR genotypes revealed that even in the setting of an HLA-matched allogeneic SCT, 63% of the patients lacked an HLA ligand for one or more donor-inhibitory KIR.18 These data support the potential for an adoptive infusion of alloreactive NK cells to reduce the incidence of GVHD and tumor relapse in humans undergoing MHC-matched T-cell–replete SCT.
Authorship
Contribution: A.L. designed and performed research, analyzed data, and wrote the paper; J.P.M. contributed vital analytical tools and analyzed data; L.S. contributed vital analytical tools and analyzed data; R.C. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard Childs, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Rm 3-5140, Bldg 10-CRC, 10 Center Dr MSC 1202, Bethesda, MD 20892-1652; e-mail: childsr@nih.nhlbi.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to acknowledge Dr Warren Shlomchik for assistance in development of the transplant model, Drs Takehito Igarashi, Yoshiyuki Takahashi, Ram Srinivasan, Maria Berg, and Anthony Suffredini for intellectual contributions related to this work, and Dante Suffredini, Sara Rosenbaum, and the Building 50 SAF staff for their excellent animal care and technical assistance.
This work was supported by the intramural research program of NIH, National Heart, Lung, and Blood Institute, Hematology Branch.

![Figure 1. Survival in murine recipients with RENCA tumors undergoing syngeneic versus MHC-matched transplantation and in vitro cytotoxicity of NK cell subpopulations against Balb/C and C57BL/6 Con-A blasts and tumor cells. Survival of Balb/C mice challenged with RENCA tumor cells that underwent SCT with allogeneic B10.d2 (⋄) or syngeneic Balb/C (♦) splenocytes (15 × 106) and bone marrow cells (8 × 106) (top panel). Images show a representative picture of lung metastases in an allogeneic (day 53) and syngeneic (day 31) transplant recipient. Arrows show RENCA tumor foci in an allogeneic transplant recipient. Lungs in syngeneic recipients were replaced by tumor nodules. Significantly higher lung weights from increased tumor burden were observed at death in recipients of syngeneic (0.70 g ± 0.07 g) vs allogeneic (0.51 g ± 0.05 g; P < .01) transplants (A). NK cells isolated from CB6F1 mice by negative depletion magnetic beads (> 95% DX5+/CD3−) were flow sorted into Ly49G2+ and Ly49C+ subpopulations, then cultured for 4 days in IL-2–containing media and stained for purity by flow cytometry (B) and tested for lytic activity against Con-A blasts and RENCA (H-2d) and B16 (H-2b) tumor cells of Balb/C (H-2d) and C57BL/6 (H-2b) origin, respectively. Ly49C+/Ly49G2− (○), Ly49G2+/Ly49C− (•), and Ly49G2+/Ly49C−/Ly49D− (▴) (C). NK cell subpopulations isolated from various strains of mice tested against Balb/C Con-A blasts and RENCA tumor cells (effector-target ratio [E/T] = 4:1) (D). Error bars depict SD of mean. One of between 3 and 5 representative experiments is shown. *P < .05 compared with opposing Ly49 NK group; unpaired t test. **The difference in survival between recipients of syngeneic and allogeneic transplantation is significant; P < .01, unpaired t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-05-024315/4/m_zh80080710950001.jpeg?Expires=1768341546&Signature=SqGXdEfCnUDEw3WPQXUBcsx8U4spQ8ALjF1dQK~lFSDYSjZQBpBLk-vwEl7snaNqhNPQXH2nQU8LZcK47lQbTpw3DMvBHRdtGC9Bto-e5DjByLQn~AYwOGlNEfKfR14eItwX5bZgDQIurCjXpW1wayLTMehZHvgzaUANJkaKUIZVfBhbMv8XBMsOTfZmnBZbKRzr5C~MOtfIfxPe92PMIkuINVQda0-uwk2YF~zYdpY1h0V3QgZ8r94YirxyNAWb-9Fu5BYS8XD8EtcmFo2yAR-TJxpE6gILW83~9Bzpq58D3wvZjOg2XhRJ7m6L3aDvDJRbOQzk5NJYKqS52VkZgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)