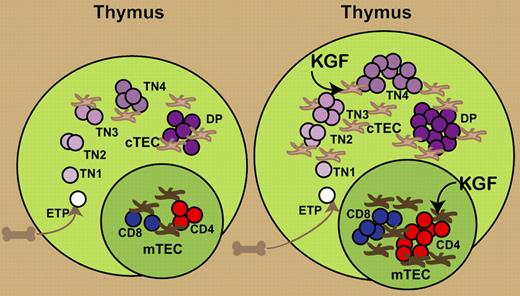
The thymic epithelium supports thymocyte development, from entry of progenitors, through T-lineage commitment, positive and negative selection, and final export. KGF boosts thymopoiesis by working directly on thymic epithelial cells.
The thymus is essential not only to the generation of a diverse and numerous T-cell population in the young, but also to replenishing that repertoire diversity throughout life. Age-dependent thymic involution diminishes thymic productive capacity, particularly limiting repopulation following severe T-cell loss. Limitations on immune reconstitution in adults result in long-term immune deficits in the elderly and in those recovering from human immunodeficiency virus (HIV) infection or cytoreductive transplant therapies.1
Increasing evidence points to the role of interactions between thymocytes and thymic epithelial cells (TECs) in determining thymic productivity. Thymic productivity is based upon the number of niches available for T-progenitor maturation and the degree of proliferative expansion occurring during maturation of these progenitors.2,3 These parameters are in turn dependent upon the thymic microenvironment. TEC populations are not static, as previously thought, but rather are constantly turning over while maintaining a stable steady-state ratio with thymocytes.4 Agents that enhance TEC numbers and function therefore may have important roles in regulating thymopoiesis.
Keratinocyte growth factor (KGF) has been found to enhance immune reconstitution after transplantation by protecting thymic structure and function from damage by radiation or graft-versus-host disease.5,6 In this issue of Blood, Rossi and colleagues present data that KGF modulates thymopoiesis through direct effects on TECs (see figure). KGF administration to intact adult mice resulted in a transient drop in thymocyte cellularity followed by a single wave of enhanced thymopoiesis. Examination of serial time points demonstrated an early proliferative wave expanding early CD3−CD4−CD8− triple-negative (TN) thymocytes, followed by successive increases in more mature thymocyte populations, culminating in enhanced export of recent thymic emigrants into the periphery.
Administration of KGF produces an increase in thymic size and cellularity. KGF directly increases the numbers of cortical and medullary TECs. This results in an increased expansion of TN thymocytes and subsequent increases in CD4+CD8+ double-positive (DP) and mature single-positive CD4 and CD8 T cells.
Administration of KGF produces an increase in thymic size and cellularity. KGF directly increases the numbers of cortical and medullary TECs. This results in an increased expansion of TN thymocytes and subsequent increases in CD4+CD8+ double-positive (DP) and mature single-positive CD4 and CD8 T cells.
The critical role of TECs in mediating the KGF effect on thymocyte development was demonstrated at several levels. MTS24+ TEC precursors and some mature cortical and medullary TECs expressed the KGF receptor, FgfR2IIIb, but thymocytes did not.6 TEC proliferation closely preceded the initial wave of TN thymocyte proliferation. Finally, adoptive transfer of early T-cell precursors (ETPs) from KGF-treated congenic mice did not increase thymic cellularity or ETP in the host, but KGF treatment of the host enhanced expansion of congenic ETPs transferred from saline-treated donors.
Rossi et al's results also shed light on the coordinated regulation of intrathymic developmental niches. The thymus imports ETPs in a gated fashion, wherein waves of intake occur only after the developmental progression of TN1 thymocytes to more mature subsets.7 ETP numbers initially decreased following KGF treatment, and increased substantially only after the initial wave of thymocyte expansion had passed. This early ETP decrease in vivo paralleled reductions in ETP uptake in KGF-treated cultures of fetal thymic lobes and concurrent reductions in fetal stromal transcripts of CCL25, a chemokine required for the homing of T progenitors. The longer-term increase observed in ETPs in KGF-treated mice may reflect an increase in the TEC niches that bind ETPs, resulting in a sustained increase in thymic productivity after the initial “wave” of thymopoiesis had passed.
Finally, the authors have begun to explore the pathways of KGF signaling in TECs. KGF increased expression of transcripts of Wnt glycoproteins and bone morphogenic proteins (BMPs) in TECs. Furthermore, in mice with knock outs of Smad4, which blocks the BMP signaling pathway, KGF did not produce an increase in thymic cellularity. These studies hold the promise of a deeper understanding of the mechanisms of KGF action on thymopoiesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ▪