The paper by Rowbotham and colleagues provides new insights into the function of the hedgehog (Hh) signaling pathway in thymocyte selection and T-cell activation.
Many of the key processes in cell fate decision, development, survival, and homeostasis are controlled by few evolutionary conserved signaling pathways including receptor tyrosine kinase, Notch, Wnt, bone morphogenetic (BMP), and the hedgehog (Hh) signaling pathways. The 3 secreted proteins of the mammalian Hh family, sonic Hh (Shh), indian Hh (Ihh), and desert Hh (Dhh), bind to the Hh receptor patched (Ptc), which in the absence of the morphogens inhibits the positive signal transducer smoothened (Smo). Smo controls various intermediate regulators that eventually lead to activation of the Gli1, Gli2, and Gli3 transcription factors. While Gli1 and Gli2 primarily act as activators, Gli3 can act both as a transcriptional activator and repressor. Aberrant Hh signaling in humans and mice is associated with a variety of developmental disorders and several types of cancer.1
Moreover, Hh signaling has been identified as an important regulatory module in T-cell development, affecting homeostasis and differentiation of early thymocyte progenitors2 and transition of double-negative (DN) cells to the double-positive (DP) stage,3 where positive and negative selection processes establish central T-cell tolerance. In the current issue of Blood, Rowbotham and colleagues have analyzed the influence of Hh signaling on the transition of immature DP cells to mature single-positive (SP) T cells. Using transgenic (Tg) mice expressing a transcriptionally active form of Gli2, the authors showed a reduction in the maturation of CD4 SP T cells, shifting the CD4/CD8 ratio toward the CD8 lineage. On the other hand, fetal thymic organ cultures (FTOCs) from Shh-deficient mice revealed a relative increase in the percentage of CD4 SP T cells. Furthermore, addition of r-Shh to FTOCs inhibited the generation of mature CD4 SP cells in wild-type and Shh−/− cultures, indicating a negative effect of Hh signaling in vivo and in vitro on the generation of CD4 SP T cells.
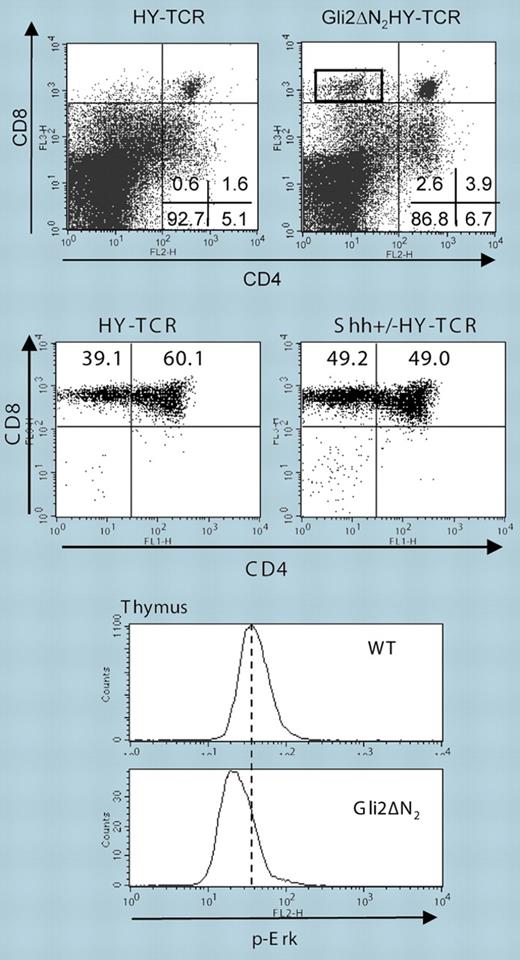
To study positive and negative selection on a defined TCR Tg background, the authors chose the HY-TCR Tg system, which allows monitoring of positive selection in female and negative selection in male mice. Expression of active Gli2 in male HY mice reduced negative selection of DP cells, leading to a 4- to 6-fold increase in the number of HY-specific CD8+ T cells in the thymus and periphery (see figure, top panel), whereas absence of Shh enhanced negative selection. Positive selection of CD8+ T cells in female HY-Gli2 double-Tg mice was not increased, but on the Shh-deficient background an enhanced selection of HY-TCR+ CD8 SP T cells became clearly apparent (see figure, middle panel). Although only one TCR Tg system was explicitly analyzed, the data provide evidence that Hh signaling is a critical parameter in the selection processes of DP cells and thus the generation of the T-cell repertoire, in the case of HY male mice fostering the maturation of “autoreactive” T cells.
Gli2ΔN2 influences Erk activation and thymocyte selection. Top panels: Gli2ΔN2 reduces negative selection in male HY-TCR Tg mice; middle panels: enhanced maturation of CD4+ T cells in female Shh+/− HY-TCR Tg mice; bottom histograms: Gli2ΔN2 Tg thymocytes show reduced activation of the MAPK Erk. See the complete figures in the article beginning on page3757.
Gli2ΔN2 influences Erk activation and thymocyte selection. Top panels: Gli2ΔN2 reduces negative selection in male HY-TCR Tg mice; middle panels: enhanced maturation of CD4+ T cells in female Shh+/− HY-TCR Tg mice; bottom histograms: Gli2ΔN2 Tg thymocytes show reduced activation of the MAPK Erk. See the complete figures in the article beginning on page3757.
DP cells have to integrate signals from both the TCR and various other surface receptors into transcriptional programs that guide positive or negative selection. Ahead lies the tremendous task of defining the biochemical and molecular mechanisms of how Gli2 and its other family members influence this signaling and transcriptional machinery. Along these lines, CD3/CD28 stimulation revealed reduced cell cycling of Gli2 Tg T cells, which was linked to a strong reduction in activation of the MAPK Erk (see figure, bottom histograms). Although how Gli2 could affect regulators of Erk activation is still an enigma, the reduction of active Erk in Gli2 Tg DP cells is of particular interest since a number of publications have shown that strength, duration, and localization of Erk are central players in thymocyte selection. The reduced expression (or downmodulation) of CD5 on DP and SP cells of Gli2 Tg mice, and, after addition of r-Shh, further indicates that Hh signaling negatively influences TCR signaling strength. Considering that Shh is expressed by epithelial cells in the thymic medulla and corticomedullary junction, one is tempted to speculate that downmodulation of TCR sensitivity via the Hh morphogens within certain thymic niches might constitute an essential event that would finalize selection and maturation processes. Finally, modulation of Erk signaling in peripheral T cells via Hh could be crucial to maintain T-cell homeostasis and to prevent the development of autoimmunity and cancerogenesis. As such and in view of prospective manipulation of T-cell responses, further insight into Gli signaling is eagerly awaited.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ▪