Abstract
Sphingosine 1-phosphate (S1P) is known to play a pivotal role in the regulation of lymphocyte emigration from organized lymphoid tissues such as the peripheral lymph nodes and thymus, but its immunologic role in unorganized and diffused tissues remains to be elucidated. Here we show that the trafficking of peritoneal B cells is principally regulated by S1P. All peritoneal B cells including B1a, B1b, and B2 B cells express comparable levels of the type 1 S1P receptor. Thus, treatment with FTY720, an S1P receptor modulator, caused the rapid disappearance of peritoneal B cells by inhibiting both their emigration from parathymic lymph nodes and their recirculation from the blood into the peritoneal cavity without affecting their progenitor populations. These changes did not affect natural plasma antibody production or phosphorylcholine (PC)–specific antibody production in serum after peritoneal immunization with heat-killed Streptococcal pneumoniae (R36A). However, FTY720 dramatically reduced peritoneal B cell-derived natural intestinal secretory IgA production without affecting the expression of J-chain and polyimmunoglobulin receptors. Additionally, FTY720 impaired the generation of PC-specific fecal IgA responses after oral immunization with R36A. These findings point to a pivotal role for S1P in connecting peritoneal B cells with intestinal B-cell immunity.
Introduction
Sphingosine 1-phosphate (S1P) has been identified as an important molecule in the regulation of lymphocyte egress from the organized lymphoid structures including the thymus and secondary lymphoid organs.1,2 At present, 5 kinds of S1P receptors have been identified, each sharing S1P as its ligand but associating with a different type of G protein, resulting in a distinct signal transduction.3 Accumulating evidence has demonstrated that type 1 S1P receptor (S1P1) is preferentially expressed on lymphocytes, and their expression is closely regulated by lymphocyte activation or development, which determines lymphocyte emigration from secondary lymph organs as well as the thymus.4,5 FTY720, 2-amino-2-[2-(4-octylphenyl)ethyl] propane-1,3-diol hydrochloride, acts as an agonist for S1P receptors, except the type 2 S1P receptor (S1P2).6–8 FTY720 blocks S1P-mediated signaling by inducing internalization of receptors.4,9 Therefore, treatment with FTY720 decreased the number of circulating lymphocytes in both blood and lymph, by inhibiting their emigration from the secondary lymphoid organs and thymus and by modulating integrin-dependent lymphocyte homing into peripheral lymph nodes.10–12
Several lines of evidence have revealed that S1P also regulates B-cell distribution in the spleen, suggesting that FTY720 can impair plasma antibody production, especially against T-dependent (TD) antigen due to the abolishment of germinal center formation.13–15 In addition, a recent study revealed that S1P plays an important role in the determination of plasma cell tropism to bone marrow.16 Despite the substantial evidence pointing to the role of S1P in the regulation of lymphocyte trafficking at the systemic immune compartments, it still remains unclear whether S1P is also involved in lymphocyte trafficking and immune responses in the mucosa-associated unorganized and diffused tissues, such as the intestinal lamina propria and the peritoneal cavity (PerC). The PerC contains numerous B cells, especially B1 B cells that can be distinguished by cell surface markers (eg, B220, IgM, IgD, CD5, and Mac-1) from conventional B2 B cells.17–19 B1 B cells are thought to play an important role in the protective immunity in PerC by producing antibodies in response to T-independent (TI) antigen, such as phosphorylcholine (PC), a haptenlike antigen associated with many pathogenic bacteria.20,21 In addition to playing a role in peritoneal immunity, B cells have been shown to be a source of IgA for the formation of secretory IgA antibody (S-IgA) in the intestine.22–24 S-IgA, a hallmark antibody principally produced at mucosal sites, plays an important role in the creation of immunologic surveillance and homeostasis at mucosa by abolishing pathogenic microbial infections and establishing symbiosis with commensal flora.25,26 One major source of S-IgA is B2 B cells derived from the common mucosal immune system (CMIS) that links inductive (eg, Peyer patches and isolated lymphoid follicles) and effector tissues (eg, lamina propria region). These B2 B cells have been shown to play a key role in the induction of TD antigen-specific S-IgA.27,28 In addition to the B cells belonging to the CMIS, peritoneal B cells have also been identified as another source of S-IgA, especially specific for the TI antigen.23,24 Accumulating evidence suggests that peritoneal B-cell trafficking is biologically regulated, in some cases by chemokines (eg, CXCL12 and CXCL13) and cytokines (eg, IL-10),29–32 but there is not currently enough information to fully understand the involvement of obvious cell trafficking molecules such as S1P in the pathway.
This study first sought to investigate the role of S1P in the regulation of peritoneal B-cell trafficking and then to assess the influence of the S1P-mediated pathway on the production of serum and intestinal antibody production from peritoneal B cells. Our current findings provide new evidence that S1P regulates peritoneal B-cell trafficking and subsequent S-IgA production in the intestine.
Materials and methods
Mice and FTY720 treatment
Female Balb/c and ICR SCID mice (7-9 weeks) were purchased from Japan Clea (Tokyo, Japan). All mice were maintained in horizontal lamina flow cabinets and provided with sterile food and water ad libitum. Mice were injected intraperitoneally with 1 mg/kg/time of FTY720 (Novartis Pharma, Basel, Switzerland).12 All animals were maintained and experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of the University of Tokyo.
Cell isolation
PerC cells were obtained by flushing the peritoneum with 8 mL ice-cold PBS.33 Lymphocytes and epithelial cells were isolated from the small intestine by the enzymatic dissociation procedure using collagenase IV (Nitta Gelatin, Osaka, Japan), as previously described.34 Lymphocytes were collected from the omentum, parathymic lymph nodes, and blood in accordance with a previously established protocol.29,35,36
Flow cytometry and cell sorting
A standard protocol, previously described, was used for flow cytometric analysis and cell sorting.37,38 Cells were first incubated with anti-CD16/32 antibody and then stained with the appropriate fluorescent-conjugated antibodies specific for CD5, CD11b, B220, IgA, and IgM (BD PharMingen, San Diego, CA). Viaprobe (BD PharMingen) was used to discriminate between dead and live cells. Flow cytometric analysis and cell sorting were performed using FACSCalibur and FACSAria (BD Biosciences, Franklin Lakes, NJ), respectively.
Quantitative and conventional RT-PCR
To measure mRNA expression for S1P receptors, quantitative reverse transcription-polymerase chain reaction (RT-PCR) using LightCycler (Roche Diagnostics, Mannheim, Germany) was performed.37 Briefly, total RNA was collected using a TRIzol reagent (Invitrogen, Carlsbad, CA) and cDNA was synthesized using Powerscript reverse transcriptase (BD Biosciences). The oligonucleotide primers and probes specific for S1P1 (forward primer, TACACTCTGACCAACAAGGA; reverse primer, ATAATGGTCTCTGGGTTGTC; FITC-probe, TGCTGGCAATTCAAGAGGCCCATCATC; LCRed 640-probe, CAGGCATGGAATTTAGCCGCAGCAAATC), S1P2 (forward primer, CATCGTACTGGGTGTTTTC; reverse primer, CCACGTATAGATGACAGGA; FITC-probe, AATAGTGGGCTTTGTAGAGGACAGGGCAGG; LCRed 640-probe, CCGAACGGGACAGGTGGAGTCTAAGAGAAG), S1P3 (forward primer, TCCTCTTCCTCATCGACGTG; reverse primer, CCTTGCCCTTGACTAGACAG; FITC-probe, TTCATCATGCTGGCTGTCCTCAACTCGG; LCRed 640-probe, CATGAACCCTGTCATCTACACGCTGGCC), S1P4 (forward primer, CATCTTTAGAGTGGTCCGAG; reverse primer, GCCCAGACATTAGAACCAA; FITC-probe, CCGCAGGCTACTCAACACCGTGCTGAT; LCRed 640-probe, ATCTTGGTGGCCTTTGTGGTGTGCTGG), and GAPDH (forward primer, TGAACGGGAAGCTCACTGG; reverse primer, TCCACCACCCTGTTGCTGTA; FITC-probe, CTGAGGACCAGGTTGTCTCCTGCGA; LCRed 640-probe, TTCAACAGCAACTCCCACTCTTCCACC) were designed and synthesized by Nihon Gene Research Laboratory (Sendai, Japan). Conventional RT-PCR was performed to measure pIgR and J-chain, using specific primers (pIgR forward, AGTATTCAGGCAGAGCCAAC; pIgR reverse, ATTCATCCGGCACAGATATT; J-chain forward, ATGAAGACCCACCTGCTTCTCTGG; J-chain reverse, AGGGTAGCAAGAATCGGGGGTCAA).
Adoptive cell transfer
For tracing cells in vivo, peritoneal cells (1 × 107 cells) were incubated with 0.25 μM CFSE (Molecular Probes, Eugene, OR) in the dark for 10 minutes at 37°C, and then washed with PBS twice in accordance with a previously described method.39 The labeled cells were transferred into severe combined immunodeficient (SCID) mice intraperitoneally (4 × 106 cells) or intravenously (1 × 107 cells) and FTY720 was simultaneously administered intraperitoneally. After 12 hours, peritoneal cells were collected for fluorescence-activated cell sorting (FACS) analysis.
For the analysis of antibody production from peritoneal B cells, SCID mice were adoptively transferred with normal peritoneal B cells (5 × 106 cells) via intraperitoneal route and treated with FTY720 every 2 days. As described under “Detection of total immunoglobulin and PC-specific antibody levels in serum and fecal extract by ELISA,” 2 weeks after the adoptive transfer, we simultaneously collected serum and fecal extracts for the measurement of total immunoglobulin levels by enzyme-linked immunosorbent assay (ELISA) and isolated mononuclear cells from the intestinal lamina propria for enumeration of antibody-forming cells (AFCs) by enzyme-linked immunospot assay (ELISPOT).
Immunization
Mice were immunized intraperitoneally with 107 heat-killed pepsin-treated Streptococcal pneumoniae strain R36A (gift of Dr John Kearney, University of Alabama, Birmingham, AL).20,29 For immunization, mice were orally immunized with 2 × 108 heat-killed pepsin-treated S pneumoniae strain R36A together with 10 μg mucosal adjuvant cholera toxin (List Biological Laboratories, Campbell, CA).40 In the FTY720-treated group, mice were injected intraperitoneally with FTY720 6 hours before the immunization and then again once per day during the experiment. Serum and fecal extracts were prepared for the analysis of PC-specific IgM and IgA production by ELISA, as described in the next section.34,38
Detection of total immunoglobulin and PC-specific antibody levels in serum and fecal extract by ELISA
Total immunoglobulin levels in serum and fecal extracts were determined by ELISA as previously described.37 To measure antibody concentration, purified murine isotype-specific antibodies (BD PharMingen) were used as standards for the quantification. For the detection of PC-specific antibodies, microtiter plates were coated with 5 μg/mL of PC-BSA (Biosearch Technologies, Novato, CA) in bicarbonate buffer (pH 9.6).29 Following blocking with 5% BSA in PBS, diluted serum or fecal extracts were added and incubated in the coated wells for 2 hours at room temperature. Bound antibodies were then determined using HRP-conjugated anti–mouse IgM or IgA (Southern Biotechnology, Birmingham, AL) and 3,3′,5,5′-tetramethylbenzidine (Moss, Pasadena, CA), as previously described.29,38
Enumeration of AFCs by ELISPOT
To measure IgM- or IgA-producing AFCs in the intestinal lamina propria, an ELISPOT assay was used as previously described.37 Briefly, various concentrations of mononuclear cells were cultured in 96-well nitrocellulose membrane plates (Millititer HA; Millipore, Bedford, MA) coated with 5 μg/mL affinity-purified goat anti-immunoglobulin (Southern Biotechnology) at 37°C for 4 hours. After vigorous washing with PBS and PBS containing 0.05% Tween 20, HRP-conjugated antibodies specific for mouse IgM or IgA (Southern Biotechnology) were added and incubated overnight. The spots of AFCs were developed using 2-amino-9-ethylcarbazole (Polysciences, Warrington, PA) containing hydrogen peroxide.
Statistics
The results were compared using a Student t test or a Welch t test. P < .05 was considered statistically significant.
Results
Rapid and reversible disappearance of peritoneal B cell by FTY720 treatment
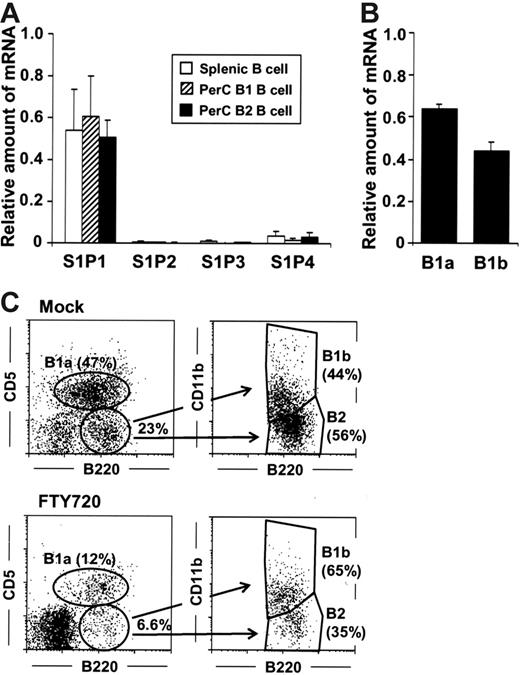
The initial aim of this study was to examine the involvement of S1P in the regulation of peritoneal B-cell trafficking. To accomplish this, we intraperitoneally administered FTY720, a modulator for S1P receptors, and examined cellular population by flow cytometry. The analysis based on forward (FSC) and side scatter (SSC) revealed that FTY720 treatment resulted in the dramatic changes in cellular population with different size and intracellular structure (Figure 1A, top panels). The profile indicated that intraperitoneal administration of FTY720 simultaneously reduced the number of lymphoid cells and induced a remarkable accumulation of granulocytes or monocytes or both (Figure 1A, top panels). Because PerC cells are known to contain both B1 and B2 B cells, we next used the expression of B220 and CD11b to confirm that numbers of both B220+CD11b+ B1 and B220+CD11b− B2 B cells were dramatically reduced (Figure 1A, bottom panels). Because the total PerC cell number remained unchanged even after FTY720 treatment (data not shown), the changes in the cellular percentage can be assumed to directly reflect the absolute cell number of each population (Figure 1B). In short, FTY720 treatment significantly decreased B1 and B2 B-cell numbers (Figure 1B).
Rapid but reversible disappearance of peritoneal B cells induced by FTY720. (A) PerC cells were isolated 10 hours after injection of FTY720 (right) or mock (left), and cell populations were analyzed using flow cytometry. The data are representative of 5 independent experiments. (B) Cell numbers of B220+CD11b+ B1 B cells and B220+CD11b− B2 B cells were calculated by using the total cell number and flow cytometric data. The error bars are ± SEM (n = 5). (C-D) At each time point after FTY720 injection, PerC cells were analyzed by flow cytometry (○, B220+CD11b+ B1 B cells; •, B220+CD11b− B2 B cells). The data represent the mean ± SD (n = 4).
Rapid but reversible disappearance of peritoneal B cells induced by FTY720. (A) PerC cells were isolated 10 hours after injection of FTY720 (right) or mock (left), and cell populations were analyzed using flow cytometry. The data are representative of 5 independent experiments. (B) Cell numbers of B220+CD11b+ B1 B cells and B220+CD11b− B2 B cells were calculated by using the total cell number and flow cytometric data. The error bars are ± SEM (n = 5). (C-D) At each time point after FTY720 injection, PerC cells were analyzed by flow cytometry (○, B220+CD11b+ B1 B cells; •, B220+CD11b− B2 B cells). The data represent the mean ± SD (n = 4).
We next sought to analyze the kinetics of the change and recovery of peritoneal B cells after treatment with FTY720. Marked reductions in both B1 and B2 B-cell levels were found only 3 hours after FTY720 treatment (Figure 1C), and a partial recovery was detected 24 hours after the injection, with full recovery observed 7 days after the administration (Figure 1D). These data suggest that the effect of FTY720 on peritoneal B cells is both rapid and reversible.
Specific and equal expression of S1P1-encoding mRNA by peritoneal B cells
As might be expected by the comparable effects of FTY720 on the removal of peritoneal B cells, both peritoneal B1 and B2 B cells were revealed by quantitative RT-PCR to express similar levels of mRNA encoding S1P1 with no or only a dim expression of the other subtype of S1P receptors (S1P2-S1P4) (Figure 2A). Because B1 B cells are further divided into B1a and B1b B cells based on CD5 expression,17–19 we then compared the S1P1 expression of B1a and B1b B cells, finding that the level of S1P1-specific mRNA expression was similar both cell groups (Figure 2B). Consistent with these observations, flow cytometric analysis revealed that FTY720 treatment resulted in roughly equivalent reductions in B220+CD11b+CD5+ B1a, B220+CD11b+CD5−B1b, and B220+CD11b−CD5−B2 B cells, indicating that the S1P-mediated pathway exerted the same degree of influence on the various types of peritoneal B cells (Figure 2C).
Equal expression of S1P1 by peritoneal B1 and B2 B cells. (A) Quantitative RT-PCR analysis for S1P receptors was performed using RNA isolated from sorted splenic B (□), peritoneal B1 (▨), and B2 (▪) B cells. The relative quantity of specific mRNA was expressed as a ratio to GAPDH. The data are expressed as mean ± SD from 4 mice. (B) S1P1 expression in B1a and B1b cells was determined by quantitative RT-PCR analysis. (C) Flow cytometric analysis was performed to characterize B1a, B1b, and B2 B cells in the PerC of mice treated with FTY720. The data are representative of 3 independent experiments.
Equal expression of S1P1 by peritoneal B1 and B2 B cells. (A) Quantitative RT-PCR analysis for S1P receptors was performed using RNA isolated from sorted splenic B (□), peritoneal B1 (▨), and B2 (▪) B cells. The relative quantity of specific mRNA was expressed as a ratio to GAPDH. The data are expressed as mean ± SD from 4 mice. (B) S1P1 expression in B1a and B1b cells was determined by quantitative RT-PCR analysis. (C) Flow cytometric analysis was performed to characterize B1a, B1b, and B2 B cells in the PerC of mice treated with FTY720. The data are representative of 3 independent experiments.
No influence of FTY720 on the differentiation and viability of peritoneal B cells
Because it has been previously reported that peritoneal B1 B cells might differentiate into monocytes such as macrophage-like cells,41 and that high concentrations of FTY720 induced lymphocyte apoptosis,42 we tested whether FTY720 induced differentiation or apoptosis of PerC cells. To address this issue, we performed in vitro culture of PerC cells with various concentrations of FTY720 or S1P. The cellular population of PerC cells such as B1 B cells, B2 B cells, and B220−CD11b+ cells (eg, macrophages) remained unaltered after 3 days of culture with biologic concentrations (1-1000 nM) of FTY720 and S1P (Figure 3A; results at 100 nM were shown). In addition, flow cytometric analysis using annexin V revealed comparable numbers of apoptotic cells in untreated and treated groups (data not shown). Together, these results indicate that FTY720 affected neither differentiation nor apoptosis of PerC cells under these experimental conditions.
FTY720 does not affect peritoneal B-cell differentiation and viability. PerC cells were cultured with 100 nM FTY720 or S1P for 3 days. Cell populations were examined by flow cytometry using antibodies specific for B220 and CD11b. Results were reproducible, with very similar data obtained from each of 3 independent experiments.
FTY720 does not affect peritoneal B-cell differentiation and viability. PerC cells were cultured with 100 nM FTY720 or S1P for 3 days. Cell populations were examined by flow cytometry using antibodies specific for B220 and CD11b. Results were reproducible, with very similar data obtained from each of 3 independent experiments.
FTY720 inhibits B-cell migration into and enhances B-cell emigration out of the PerC
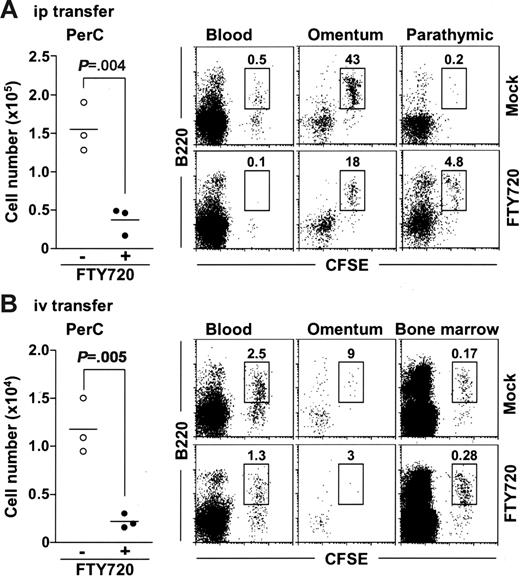
To determine whether FTY720 reduced peritoneal B cells by promoting their emigration from the PerC, or by inhibiting their migration into the PerC, or both, we isolated peritoneal B cells from normal mice, labeled them with 5- (and -6)–carboxyfluorescein diacetate succinimidyl ester (CFSE), and adoptively transferred them into the PerC of SCID mice and compared their emigration in animals receiving FTY720 treatment and in those that did not. We found that FTY720 treatment significantly decreased the numbers of peritoneal CFSE+ cells in the PerC of treated mice (Figure 4A). We then addressed whether the transferred B cells were present in the circulation or migrated to other tissues. We barely detected CFSE+ cells in the blood of FTY720-treated mice (Figure 4A). Because it has been considered that lymphocytes pass through the omentum and parathymic lymph nodes on their way to the blood from the PerC,35,36 we next examined the cell population in these tissues. Flow cytometric analysis revealed that the number of CFSE+ B220+ cells was reduced in the omentum but increased in the parathymic lymph nodes of FTY720-treated mice (Figure 4A). These data indicate that FTY720 treatment induces the accumulation of peritoneal B cells in the parathymic lymph nodes, leading to a reduction of these cells in the PerC and the blood.
FTY720 simultaneously inhibits their emigration from the parathymic lymph nodes and their entrance from the blood into the PerC. SCID mice were adoptively transferred with CFSE-labeled normal PerC B cells via the intraperitoneal (A) or the intravenous (B) route, and simultaneously treated with (• or bottom panels) or without (○ or top panels) FTY720. After 12 hours, cells were isolated from the PerC, blood, omentum, parathymic lymph nodes, and bone marrow for the analysis of CFSE+ cells. Horizontal bars represent the mean.
FTY720 simultaneously inhibits their emigration from the parathymic lymph nodes and their entrance from the blood into the PerC. SCID mice were adoptively transferred with CFSE-labeled normal PerC B cells via the intraperitoneal (A) or the intravenous (B) route, and simultaneously treated with (• or bottom panels) or without (○ or top panels) FTY720. After 12 hours, cells were isolated from the PerC, blood, omentum, parathymic lymph nodes, and bone marrow for the analysis of CFSE+ cells. Horizontal bars represent the mean.
Under similar experiments, we also examined the effect of FTY720 on their migration from the blood into the PerC because it was previously reported that mature B cells could home to the PerC from the blood.29 We transferred CFSE-labeled peritoneal cells to SCID mice via the intravenous route and compared their migration into PerC of mice receiving FTY720 and in those that did not. The number of CFSE+ cells was significantly lower in the PerC when mice were treated with FTY720, indicating that FTY720 inhibited the migration of peritoneal B cells from the blood circulation into the PerC (Figure 4B). In these mice, CFSE+ cell numbers were reduced in the blood, omentum, and parathymic lymph nodes but increased in the bone marrow (Figure 4B and data not shown). These data suggest that FTY720 directs the circulating B cells to migrate to the bone marrow rather than to the PerC. Collectively, these findings indicate that FTY720 removes the peritoneal B cells both by inhibiting their emigration from the parathymic lymph nodes and by changing the tropism of circulating B cells to bone marrow.
No influence of FTY720 treatment on B1 B-cell progenitors in the PerC
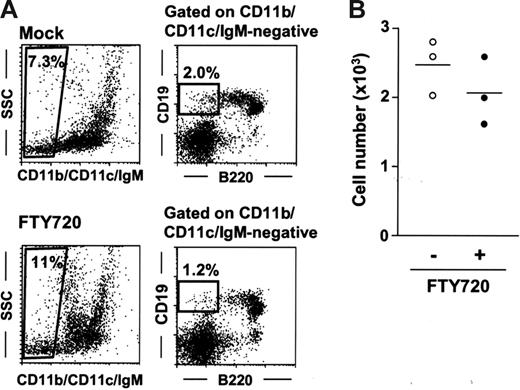
Having established that the effects of FTY720 on peritoneal B cells were rapid and reversible (Figure 1), we next sought to determine whether FTY720 influenced peritoneal B-cell development. To address this issue, we investigated the B1 B-cell progenitor population (CD11b−IgM−B220low/negCD19+) recently identified in the bone marrow.43 In this study, we found the presence of CD11b−CD11c−IgM−B220low/negCD19+ cells in the PerC at rates of 2.5 × 103 cells/mouse (0.1% in total peritoneal cells; Figure 5A-B). Treatment with FTY720 did not appreciably affect progenitor numbers in either the PerC (Figure 5A-B) or the bone marrow (data not shown).
No influence of FTY720 treatment on peritoneal B1 B-cell progenitor. (A) B1 B-cell progenitors were determined as CD11b−CD11c−IgM−B220int-negCD19+ cells. These cells were isolated from the PerC 6 hours after mock (top panels) or FTY720 (bottom panels) treatment. Data are representative of 3 independent experiments. (B) The number of peritoneal CD11b−CD11c− IgM−B220int-negCD19+ cells was calculated by using the total cell number and the flow cytometric data. Horizontal bars represent the mean.
No influence of FTY720 treatment on peritoneal B1 B-cell progenitor. (A) B1 B-cell progenitors were determined as CD11b−CD11c−IgM−B220int-negCD19+ cells. These cells were isolated from the PerC 6 hours after mock (top panels) or FTY720 (bottom panels) treatment. Data are representative of 3 independent experiments. (B) The number of peritoneal CD11b−CD11c− IgM−B220int-negCD19+ cells was calculated by using the total cell number and the flow cytometric data. Horizontal bars represent the mean.
Comparable serum antibody production under natural conditions and after immunization with bacterial antigen
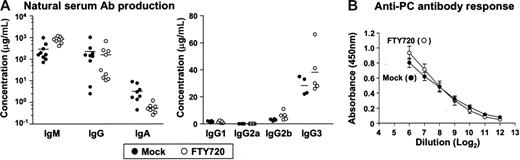
In the next experiment, we set out to determine whether the FTY720-induced disappearance of peritoneal B cells exerted any influence on systemic and mucosal antibody production. Because peritoneal B cells are well characterized as a source of natural antibody production,17–19 we examined the total serum antibody production in SCID mice following adoptive transfer of normal peritoneal cells and continuous treatment with FTY720. Comparable productions of serum IgG and IgM were detected in mock- and FTY720-treated mice, whereas serum IgA production decreased partially in mice treated with FTY720 (Figure 6A). FTY720 did not affect IgG subclasses, and so TI antigen-associated subclasses of IgG2b and IgG3 were prevalent in both mock- and FTY720-treated mice (Figure 6A). These data indicate that FTY720 induces B1 B-cell alteration in the PerC but does not affect the generation of natural serum antibody production.
Effects of FTY720 on serum antibody production. (A) SCID mice were adoptively transferred with 5 × 106 normal PerC B cells and were treated with mock (•) or FTY720 (○) every 2 days. Two weeks after the transfer, serum was collected for the measurement of total immunoglobulin levels by ELISA. Horizontal bars represent the mean. (B) Mice pretreated with FTY720 and intraperitoneally immunized with heat-killed, pepsin-treated S pneumoniae strain R36A, received daily treatment with FTY720. After 5 days, serum anti-PC IgM was measured by ELISA. The error bars are ± SEM (n = 4) from 2 separate experiments.
Effects of FTY720 on serum antibody production. (A) SCID mice were adoptively transferred with 5 × 106 normal PerC B cells and were treated with mock (•) or FTY720 (○) every 2 days. Two weeks after the transfer, serum was collected for the measurement of total immunoglobulin levels by ELISA. Horizontal bars represent the mean. (B) Mice pretreated with FTY720 and intraperitoneally immunized with heat-killed, pepsin-treated S pneumoniae strain R36A, received daily treatment with FTY720. After 5 days, serum anti-PC IgM was measured by ELISA. The error bars are ± SEM (n = 4) from 2 separate experiments.
To investigate the effects of FTY720 on the induction of bacterial antigen-specific antibody production, we used PC, a main TI antigen on the bacterial wall, as a model antigen, since B1 B cells have been shown to be a major source of PC-specific antibodies.20,29 Accordingly, we intraperitoneally immunized mock- or FTY720-pretreated mice with R36A, a heat-killed, pepsin-treated S pneumoniae strain20,29 and continued to treat every day for 5 days with mock or FTY720, respectively. Although repeated treatment with FTY720 was meant to maintain the low number of peritoneal B1 B cells during the experiment, in the end similar levels of PC-specific IgM production were detected in both mock- and FTY720-treated mice (Figure 6B). These findings suggest that the alteration of B-cell trafficking induced by FTY720 in the PerC did not affect either natural or bacterial antigen-specific serum antibody production.
Reduction of intestinal IgA production by treatment with FTY720
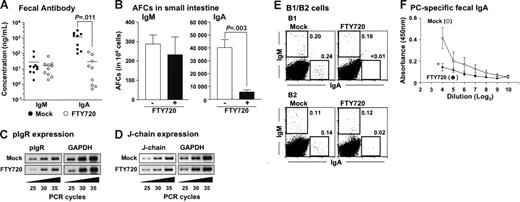
Finally, we investigated whether the alteration of peritoneal B-cell trafficking affected intestinal antibody production since peritoneal B cells are thought to migrate into intestinal lamina propria and contribute to subsequent IgA production.22–24 When peritoneal B cells were adoptively transferred into SCID mice, considerable IgA and IgM production was noted in the feces (Figure 7A) and consistent with such antibody production, IgA or IgM AFCs were detected in the lamina propria region (Figure 7B). In contrast, when SCID mice were continuously treated with FTY720 after the adoptive transfer of peritoneal B cells, fecal IgA production was significantly impaired in FTY720-treated mice, whereas fecal IgM production was comparable to that seen in the mock-treated mice (Figure 7A). ELISPOT analysis confirmed this finding, demonstrating that IgA AFCs levels were significantly reduced in the intestinal lamina propria of mice treated with FTY720, whereas IgM AFCs levels were to mock-treated group (Figure 7B). Additionally, RT-PCR analysis revealed that mock- and FTY720-treated mice expressed identical polyimmunoglobulin receptors (pIgRs) in intestinal epithelial cells and J-chain in intestinal B cells. Thus, the reduction of fecal IgA could not be due to a defect in the formation of polymeric IgA or its subsequent transport via epithelial cells into the lumen (Figures 7C-D).
Impaired fecal IgA production after treatment with FTY720. (A) Fecal extracts were collected from the reconstituted SCID mice and analyzed for immunoglobulin production by ELISA as described in Figure 6A. Horizontal bars represent the mean. (B) Similarly, mononuclear cells were isolated from small intestinal lamina propria and used for the ELISPOT assay. The error bars are ± SEM (n = 5). (C-D) pIgR expression in epithelial cells (C) and J-chain expression in lamina propria lymphocytes (D) were examined by RT-PCR. Data are representative of 3 independent experiments. (E) SCID mice were adoptively transferred with purified peritoneal B1 or B2 cells and treated with FTY720 as described in Figure 6A. FACS analysis was performed to detect IgA+ and IgM+ cells in the intestinal lamina propria of mice treated with (right) or without (left) FTY720. Data are representative of 3 independent experiments. (F) Mice were orally immunized with R36A together with cholera toxin and treated with (•) or without (○) FTY720. After 3 days, fecal PC-specific IgA levels were measured by ELISA. The error bars are ± SEM (n = 4). *P < .05.
Impaired fecal IgA production after treatment with FTY720. (A) Fecal extracts were collected from the reconstituted SCID mice and analyzed for immunoglobulin production by ELISA as described in Figure 6A. Horizontal bars represent the mean. (B) Similarly, mononuclear cells were isolated from small intestinal lamina propria and used for the ELISPOT assay. The error bars are ± SEM (n = 5). (C-D) pIgR expression in epithelial cells (C) and J-chain expression in lamina propria lymphocytes (D) were examined by RT-PCR. Data are representative of 3 independent experiments. (E) SCID mice were adoptively transferred with purified peritoneal B1 or B2 cells and treated with FTY720 as described in Figure 6A. FACS analysis was performed to detect IgA+ and IgM+ cells in the intestinal lamina propria of mice treated with (right) or without (left) FTY720. Data are representative of 3 independent experiments. (F) Mice were orally immunized with R36A together with cholera toxin and treated with (•) or without (○) FTY720. After 3 days, fecal PC-specific IgA levels were measured by ELISA. The error bars are ± SEM (n = 4). *P < .05.
We next examined the contribution, if any, made to S1P-dependent intestinal IgA production by B1 and B2 B cells. To address this issue, we adoptively transferred purified peritoneal B1 or B2 B cells into SCID mice, treated with or without FTY720, and then examined intestinal B cells. Flow cytometric analysis revealed that both B1 and B2 B cells equally developed IgA+ and IgM+ cells in the intestine (Figure 7E). As might be expected from the observed selective reduction in intestinal IgA production after FTY720 treatment (Figure 7A-B), FTY720 was found to reduce IgA+ cells regardless of the subset of B cells from which they originated, suggesting that FTY720 equally affects B1 and B2 B cells. Thus, both B1 and B2 B cell-derived IgA+ cells were equally decreased in the intestine of FTY720-treated mice (Figure 7E). These findings were further confirmed by showing that FTY720 treatment reduced both B1- and B2-derived fecal IgA production (data not shown). Additionally, PC-specific fecal IgA production was impaired when mice received FTY720 after oral immunization with R36A (Figure 7F). These results suggest that the cell trafficking of intestinal IgA-committed B1 and B2 B cells from the PerC is under the regulation of S1P.
Discussion
S1P is principally produced by platelets during platelet activation and thrombotic processes.44 S1P concentrations in serum are stably maintained (100-300 nM concentration) by binding with serum protein (eg, albumin) and enzymatic degradation.45–47 However, S1P receptors are biologically regulated and so more given to fluctuation.2,48 It has been well demonstrated that S1P1 is preferentially expressed on the lymphocytes and that its expression is altered during lymphocyte development and activation, a process that plays an important role in the regulation of lymphocyte egress from the organized lymphoid structures including the thymus and secondary lymphoid organs.4,5 However, its immunologic role in the regulation of lymphocyte trafficking at nonorganized tissues such as the PerC and the intestinal lamina propria region remains less than fully understood. In this study, we demonstrated that the interaction between S1P and its receptor (S1P1) also regulated peritoneal B-cell trafficking, both inbound and outbound. Despite their immunologic differences,18,21,49 B1a, B1b, and B2 B cells in the PerC expressed similar levels of S1P1 expression and exhibited a comparable dependency on S1P (Figures 1-2). These comparable involvements of S1P-mediated pathway were different from chemokine-mediate pathway. For instance, CXCL13/CXCR5- or CCR7-mediated pathways showed different regulation ability against peritoneal B1 and B2 B cell.29,30 The difference might instead be explained by the previously demonstrated hierarchy that exists between S1P and chemokines.13 The study showed that S1P signaling overcame the recruiting activity of CXCL13 in the regulation of marginal zone B-cell localization.13 Because CXCL13 has been reported to play the major role in the B-cell retention in the PerC,29 it is likely that S1P simultaneously overcomes the CXCL13-mediated retention of peritoneal B cells and enhances the emigration of B cells out of the peritoneal cavity.
In addition, adoptive transfer experiments also revealed that FTY720 removed peritoneal B cells via at least 2 distinct pathways (Figure 4): (1) inhibition of their egress out of the parathymic lymph nodes on their way to the blood circulation from the PerC and (2) their migration from the blood into the PerC. The first observation accords well with current thinking that lymphocytes can traffic from the peritoneal cavity to the blood through omentum and parathymic lymph nodes35,36 and that cells accumulate in the lymph nodes after FTY720 treatment.10–12 Although it has been demonstrated that FTY720 treatment induces an accumulation of naïve B cell in the bone marrow,10 we demonstrate here for the first time that S1P also regulates the immigration of mature B cells from the blood circulation into the PerC. These results lend support to the recent contention by Butcher's group that lymphocyte egress from structurally nonlymphoid tissues (eg, skin) is not random, but biologically regulated and that CCR7-mediated signaling plays an important role in that regulation.39 Our current findings point to S1P as another key molecule regulating lymphocyte trafficking in unorganized and diffused tissues (eg, the PerC).
Although the precise developmental pathway for peritoneal B cells remains controversial, it is generally thought that peritoneal B1 B cells originate from fetal liver,50 fetal omentum,51 and para-aortic splanchnopleura,52 whereas B2 cells are preferentially generated from bone marrow.53 In addition, a recent study has identified B1 B progenitor cells in bone marrow.43 These progenitor cells are characterized as Lin− CD19+ B220low-neg and have the ability to differentiate into either peritoneal B1a or B1b cells.43 In this study, we found similar CD11b−CD11c−IgM−CD19+-B220low-neg cells in the PerC (Figure 5). Although it is still unclear whether peritoneal CD11b−CD11c−IgM−CD19+B220low-neg cells are derived from the bone marrow or from other sites (eg, fetal liver), it is interesting to note that FTY720 removed a marked number of peritoneal B220+ B cells, but did not affect CD11b−CD11c−IgM−CD19+B220low-neg cell numbers (Figure 5). Together with a previous study demonstrating that S1P1 expression is up-regulated during T-cell development in the thymus,4 our current results raise the possibility that S1P1 expression is similarly up-regulated during B-cell development in PerC, perhaps accounting for the fact that FTY720 affects B220+ PerC cells, but not CD11b−CD11c− IgM−CD19+B220low-neg cells (Figures 1-2 and 5).
FTY720 treatment diminishes germinal center formation and so drastically curtails antibody production against the TD antigen, but it does not affect antibody production against TI antigen.14,15 Consistent with these reports, FTY720 treatment of SCID mice adoptively transferred with normal PerC cells did not influence the natural antibody production in serum. Thus, comparable levels of total plasma IgG production, mainly IgG2b and IgG3, were detected in mock- and FTY720-treated mice (Figure 6A). Since we transferred PerC cells into SCID mice lacking functional T cells, the preferential production of IgG2b and IgG3, well-known subclasses dominantly reactive to TI antigen, was expected. We also found that FTY720 did not affect anti-PC IgM production after immunization of normal mice with heat-killed, pepsin-treated S pneumoniae strain, R36A (Figure 6B). This observation was consistent with previous reports demonstrating that FTY720 did not influence antibody production against soluble TI antigen (eg, TNP-Ficoll and NP-Ficoll)14,15 and further suggested that FTY720-mediated alteration of peritoneal B-cell distribution did not affect antibody production against TI antigen regardless of antigen form (eg, soluble or particulate). However, this observation contradicted a previous report demonstrating that peritoneal B-cell paucity in CXCL13-deficient mice resulted in the impaired antibody responses against intraperitoneal immunization of S pneumoniae.29 A variety of scenarios could account for this discrepancy. Our findings showed that the peritoneal B1 and B2 B cells never completely disappeared, even after repeated administration of FTY720 (Figures 1-2 and data not shown). Those remaining cells are nonreactive to FTY720 and might contribute to antibody production against S pneumoniae. Using chemokine receptor expression (CXCR5 and CCR7) to distinguish among B-cell populations and to identify the nonreactive population in the PerC, we compared the effect of FTY720 on the cells types and did not find any differences (data not shown). In addition, based on the recent report that CD69 induced the internalization of S1P1 and negatively regulated S1P-mediated signaling,54 we sought to examine the CD69 expression of peritoneal B cells, demonstrating that peritoneal B cells were exclusively CD69 negative (data not shown). Similarly, no difference was detected in surface immunoglobulin expression (data not shown). Alternatively, though a small dose of bacterial antigen (107) was intraperitoneally administered, intact or processed bacterial antigen could be carried into other immunologic sites (eg, spleen, intestine, and bone marrow) by peritoneal macrophages or dendritic cells and activated B cells for the production of PC-specific IgM.
Although the serum antibody production originating peritoneal B cells was unaltered in FTY720-treated mice, peritoneal B1 and B2 B cell–derived intestinal S-IgA production and IgA AFCs in the intestinal lamina propria were markedly reduced after FTY720 treatment and so shown to be under the regulation of S1P. S-IgA production was significantly reduced (150 times less than mock-treated mice), but IgM levels remained largely unaffected (Figure 7A-B). However, FTY720 treatment did not affect pIgR and J-chain expression (Figure 7C-D). Again, several scenarios could account for these findings. First, since previous studies proposed the mutual interaction between S1P- and chemokine-mediated pathway in the lymphocyte trafficking,13,16,55–57 the cooperative pathway mediated by both S1P and chemokine may determine the selective effects of FTY720 on IgA+ B cells. These selective effects could be mediated by the differing degree of dependency by IgM+ B cells and IgA+ B cells on S1P for the migration of peritoneal B cells into the intestine. In this regard, it was reported that CCR10 expression was prevalent on IgA+ B cells with a plasmablast and plasma cell phenotype in the blood and the intestine but negligible on IgA− B cells.58 We found that intraperitoneally transferred CFSE+ B cells were barely detected in the blood but some CFSE+ B cells were still present in the omentum, which adjoins the gastrointestinal compartment (Figure 4A).35,36 This finding led us to consider a second scenario, namely, that peritoneal B cells have a unique S1P-independent trafficking pathway from the PerC to the intestine through the omentum. Yet a third possibility is that FTY720 inhibited class switch recombination from IgM to IgA and thereby the subsequent differentiation into IgA plasma cells. Similar phenomena were observed in mice lacking activation-induced cytidine deaminase (AID), an essential molecule for class switch recombination.59 A previous study demonstrating that sphingosine inhibited IL-5–induced IgA synthesis in LPS-stimulated murine B cells lends credibility to a fourth possibility: that FTY720 induces down-regulation of IgA production.60 These possibilities may similarly account for the discrepancy that FTY720 reduced PC-specific fecal IgA production but not PC-specific serum IgM after immunization with R36A (Figures 5B and 7F). We are currently engaged in studies to clarify this issue.
In summary, we have demonstrated that S1P plays an important role in the regulation of lymphocyte trafficking not only in the organized lymphoid tissues, but also in the unorganized and diffused tissues. It also plays a pivotal role in linking peritoneal B cells to intestinal IgA production. Collectively, our findings point to a novel immunologic significance for S1P in CMIS-independent mucosal immunity.
Authorship
Contribution: J.K. designed research, performed research, analyzed data, and wrote the paper; Y.K., M.G., M.H., I.I., F.M., and I.O. performed research and analyzed data; and H.K. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiroshi Kiyono, Division of Mucosal Immunology, Department of Microbiology and Immunology, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: kiyono@ims.u-tokyo.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Corporation (JST); the Ministry of Education, Science, Sports, and Culture; and the Ministry of Health and Welfare in Japan.
We thank Dr J. Kearney (University of Alabama, Birmingham) for reagents and helpful discussion. We also appreciate Dr K. McGhee for editorial help and Novartis Pharma for providing FTY720.