Abstract
Dendritic cells (DCs) have the unique ability to efficiently present T-cell epitopes from exogenous antigens on MHC class I molecules, a process called cross-presentation. In our study we demonstrate that stimulation of monocyte-derived DCs with Toll-like receptor (TLR) ligands differentially affects the uptake and cross-presentation of cellular antigens. Activation of DCs with TLR3 or TLR4 but not with TLR2 or TLR7/8 ligands inhibited phagocytosis of apoptotic tumor cells and resulted in a reduced cross-presentation of pp65-derived T-cell epitopes on MHC class I molecules upon engulfment of cytomegalovirus (CMV)–infected fibroblasts. These results have an important impact on the understanding of the interactions between the immune system and pathogens and the development of vaccination strategies to treat malignant diseases.
Introduction
Dendritic cells (DCs) are recognized as the most efficient antigen-presenting cells that initiate antigen-specific immune responses. They reside in an immature state in peripheral tissues, where they sense their environment and take up antigens. Upon activation through inflammatory mediators or pathogen-derived products, they change their expression pattern of various cell-surface molecules and secreted mediators like cytokines and chemokines. These alterations enable DCs to migrate to lymphoid tissues, where they present antigens and induce differentiation of both naive CD4+ and CD8+ T lymphocytes. These functional changes are accompanied with a lower capacity to take up soluble and cellular antigens by phagocytosis or pinocytosis by mature DCs and an increased ability to stimulate T-cell responses.1,2
Usually, antigens from exogenous sources are taken up by DCs, become processed, and are presented to CD4+ T lymphocytes on MHC class II molecules while intracellular-derived peptides are loaded onto MHC class I molecules where they stimulate antigen-specific CD8+ T lymphocytes.3 However, DCs are able to present antigens taken up from their environment on MHC class I molecules in a process called cross-presentation. This mechanism enables these cells to raise immune responses against tumor cells or pathogens like viruses that do not infect themselves.4–8
DCs employ various molecules to sense their environment for pathogen-associated molecular patterns (PAMPs), microbial elementary components that are not or only minimal subjected to host adaptation. The most prominent of these receptors resembles the Toll-like receptor (TLR) family. TLR family members recognize microbial components such as lipopolysaccharide (LPS), flagellin, lipopeptides, or nucleic acids, and therefore initiate specific signaling pathways that lead to distinct immune responses.9
In our study, we analyzed the effects of TLR-mediated DC activation on uptake of cellular antigens and their cross-presentation on MHC class I molecules. We show that TLR3 or TLR4 but not TLR2 or TLR7/8 matured DCs are impaired in the phagocytosis of cellular material and subsequent cross-presentation of antigens on MHC class I molecules.
Materials and methods
Approval was obtained from the ethics committee of the University of Tübingen for these studies. In our study we used buffy coats provided by the local blood bank. Informed consent was provided according to the Declaration of Helsinki.
Cells
HEK-293 cells (embryonal kidney; DSMZ, Braunschweig, Germany) were cultured in RP10 medium (RPMI 1640 with glutamax I, supplemented with 10% inactivated fetal calf serum [FCS] and antibiotics; Invitrogen, Karlsruhe, Germany).
Generation of DCs
DCs were generated from peripheral blood monocytes by magnetic cell sorting as described previously.10 In brief, peripheral blood mononuclear cells were isolated by Ficoll/Paque (Biochrom, Berlin, Germany) density gradient centrifugation of blood obtained from buffy coats of healthy volunteers from the blood bank of the University of Tübingen. For magnetic cell sorting, CD14 magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were used and purities of more than 95% were achieved. Monocytes were plated (1 × 106 cells/3 mL per well) into 6-well plates (BD-Falcon, Heidelberg, Germany) in RP10 medium supplemented with granulocyte macrophage–colony-stimulating factor (GM-CSF, 100 ng/mL; Sargramostim; Berlex, Richmond, VA) and interleukin 4 (IL-4, 20 ng/mL; R&D Systems, Wiesbaden, Germany). Cytokines were added to differentiating DCs every 2 to 3 days. DC maturation was induced on day 6 by adding one or a combination of 2 of the following TLR ligands: Pam3Cys (TLR2L, 5 μg/mL; EMC Microcollection, Tübingen, Germany), Poly I:C (TLR3L, 50 μg/mL; Sigma, Deisenhofen, Germany), LPS (TLR4L, 100 ng/mL; Sigma), or R848 (TLR7/8L, 2 μg/mL; InvivoGen, San Diego, CA), respectively.
Immunostaining
Phenotypic analysis was done through fluorescence activated cell sorting (FACS). Cells were stained using fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated mouse monoclonal antibodies against CD14, CD80, HLA-DR, CD54 (BD Biosciences, Heidelberg, Germany); CD40, CD86 (BD Pharmingen, Heidelberg, Germany); CD1a, HLA-ABC (Dako Diagnostika GmbH, Hamburg, Germany); CD83 (Immunotech, Marseille, France); and mouse IgG isotype control (BD Biosciences). Cells were analyzed on a FACSCalibur cytometer (BD Biosciences). To calculate the percentage of positive cells, a proportion of 1% false-positive events was accepted in the negative control samples throughout.
Induction of apoptosis
Apoptosis was induced by resuspending HEK-293 cells in phosphate-buffered saline (PBS) followed by UV-irradiation in petri dishes with 100 mJ on a Stratalinker 2400 (Stratagene, Amsterdam, The Netherlands). Cells were immediately transferred in RP10-medium for overnight cultivation and were harvested the next day for annexin V/propidium iodide (PI) staining and phagocytosis experiments.
Phagocytosis assays
To determine the uptake of apoptotic cells by DCs, DCs and HEK-293 cells were labeled with Paul Karl Horan 67 (PKH67; green) or PKH26 (red) membrane dyes, respectively, according to the manufacturer's instructions (Sigma). In the case of HEK-293 cells, staining was done after UV irradiation. For phagocytosis, 1 × 105 DCs and 1 × 105 HEK-293 cells were cocultivated in 24-well plates in 1 mL RP10 medium. After incubation for 24 hours at 37°C, cells were harvested and analyzed by flow cytometry. For confocal laser scanning microscopy, cells were coated on slides and were analyzed on a Leica TCS SP (Leica Microsystems, Bensheim, Germany), equipped with an HCX PL 63×/1.32-0.60 numeric aperture oil objective. Images were taken using Leica PowerScan Software TCS SP1.
CMV infection of fibroblasts
CMV infection of fibroblasts was performed as described previously.11 In brief, HLA-A2–negative human foreskin fibroblasts (HFFs) were cultured in minimum essential medium (MEM; Invitrogen) containing 2.4 mM glutamine, 100 mg/mL gentamycin, and 5% FCS. Fibroblasts were used for experiments between passages 10 and 25. For preparation of virus stocks, HFFs were infected at a multiplicity of infection of 0.1 with human CMV strain AD169. Supernatants of infected cultures were harvested 6 days after infection, cells and cell debris were removed by centrifugation, and cell-free virus preparations were then used for infection of HFF cultures. The medium was removed and replaced by virus preparations for 120 minutes at 37°C. Subsequently, virus preparations were removed and cells were washed with fresh medium and used for immunologic assays. Furthermore, to exclude unspecific loading with CMV antigens, DCs were cultivated for 120 minutes at 37°C with the supernatant from the fibroblast infection.
IFN-γ ELIspot assay
A CMV-specific HLA-A2+ cytotoxic T-lymphocyte (CTL) line was generated in vitro using autologous DCs loaded with the HLA-A2–specific pp65-peptide NLVPMVATV (amino acids 495-503; A*0201), as described previously.11,12 After one restimulation cells were collected and incubated at a concentration of 5 × 104 or 1 × 105 cells/well in an anti-human IFN-γ antibody (10 μg/mL; Hölzel Diagnostika, Cologne, Germany)–coated 96-well plate with 1 × 104 autologous DCs. As positive or negative controls, autologous DCs were pulsed with the specific CMV peptide or an irrelevant HLA-A2–binding peptide derived from HIV (ILKEPVHGV; amino acids: 476-484; pol HIV-1 reverse transcriptase), respectively. Furthermore, autologous DCs that were cocultivated with irradiated (96 Gy, Gammacell 1000; Atomic Energy of Canada, Ontario, Canada) CMV-uninfected or -infected HFFs were used as stimulators. After 48 hours of incubation, visualization of IFN-γ release was done using a biotin-labeled anti-human IFN-γ antibody (anti-human IFN-γ Biotin, 2 μg/ml; Hölzel Diagnostika). Spots were counted using an automated ELIspot reader (Immunospot Analyzer; CTL Analyzers LLC, Cleveland, OH).
Results
Apoptotic HEK-293 cells are phagocytosed by in vitro–cultured DCs
Clearing of apoptotic cells or cell fragments is an essential and well-investigated function of DCs.7,13 To analyze the effect of TLR ligands on DC maturation and their capability to engulf cellular material, we established an in vitro assay to directly measure phagocytic activity. At first, different human tumor cell lines were tested for their sensitivity to UV irradiation and subsequent induction of apoptosis (data not shown). One of these, the HEK-293 cell line, was chosen for further experiments due to its susceptibility to UV light and its subsequent induction of apoptosis, as analyzed by annexin V/PI stainings (Figure S1, available on the Blood web site; see the Supplemental Figures link at the top of the online article).
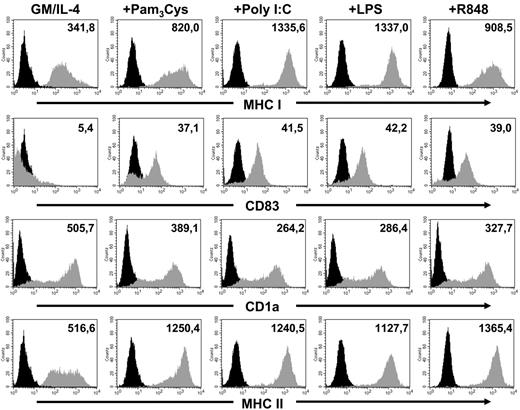
DCs were generated in vitro from CD14+ peripheral blood monocytes from healthy donors using GM-CSF and IL-4. On day 6, the cells were stimulated for 24 hours with different TLR ligands or were left untreated. The next day cells were phenotypically analyzed for the typical morphology with dendrite shape and surface expression of CD1a and HLA-DR accompanied by the absence of CD14 as analyzed by FACS. Furthermore, activation of DCs led to an up-regulation of CD83, CD80, and CD86 (Figure 1 and data not shown).
Analysis of the DC phenotype. Immature DCs or DCs matured with different TLR ligands were phenotypically analyzed by flow cytometry. Stainings were done with monoclonal antibodies recognizing CD1a, CD83, and MHC class I and class II. Gray histograms represent stainings with the indicated antibody, black histograms represent the isotype control. The level of surface expression is indicated as mean fluorescence intensity.
Analysis of the DC phenotype. Immature DCs or DCs matured with different TLR ligands were phenotypically analyzed by flow cytometry. Stainings were done with monoclonal antibodies recognizing CD1a, CD83, and MHC class I and class II. Gray histograms represent stainings with the indicated antibody, black histograms represent the isotype control. The level of surface expression is indicated as mean fluorescence intensity.
To analyze the capability of differentially activated DCs to take up apoptotic cells or cell fragments, a phagocytosis experiment was established. Immature DCs and apoptotic HEK-293 cells were labeled with different PKH-membrane dyes and were subsequently cocultivated for different time periods (Figure S2 and data not shown). Uptake of apoptotic cells is represented by the double-positive cell fraction in FACS analysis. As a control, phagocytosis was performed at 4°C and, in addition, labeled cells were mixed shortly before FACS analysis to exclude unspecific diffusion of membrane dyes (Figure S2C, E). Furthermore, to exclude signals caused by unspecific binding of apoptotic material on the cell surface of DCs, confocal laser scanning microscopy was performed (Figure S3).
TLR-activated DCs differentially take up antigens
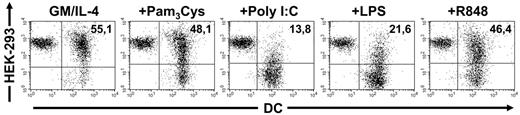
To analyze the impact of different TLR ligands on the uptake of apoptotic cells by DCs, maturation of these cells was triggered using Pam3Cys (TLR2 ligand), Poly I:C (TLR3 ligand), LPS (TLR4 ligand), or R848 (TLR7/8 ligand). After subsequent labeling of the cells with membrane dyes, cocultivation was performed for another 24 hours. As expected, immature DCs showed a strong phagocytic activity and uptake of large amounts of apoptotic cells could be observed (shift to red; Figure 2). Surprisingly, Pam3Cys- and R848-activated DCs displayed a phagocytic rate similar to that found in immature DCs. In contrast, only the Poly I:C- and LPS-treated DCs exhibited a diminished uptake of apoptotic cells.
TLR-activated DCs differentially take up apoptotic cells. The ability of differentially stimulated DCs to take up apoptotic material was analyzed in coculture experiments. TLR3 (Poly I:C)– and TLR4 (LPS)–stimulated DCs are impaired in the phagocytosis of cellular material whereas TLR2 (Pam3Cys) and TLR7 (R848) activation do not significantly alter this property in comparison to immature DCs. Percentage of double-positive cells is shown in the upper right quadrant. The data shown are representative of 5 independent experiments.
TLR-activated DCs differentially take up apoptotic cells. The ability of differentially stimulated DCs to take up apoptotic material was analyzed in coculture experiments. TLR3 (Poly I:C)– and TLR4 (LPS)–stimulated DCs are impaired in the phagocytosis of cellular material whereas TLR2 (Pam3Cys) and TLR7 (R848) activation do not significantly alter this property in comparison to immature DCs. Percentage of double-positive cells is shown in the upper right quadrant. The data shown are representative of 5 independent experiments.
CMV-derived antigens are cross-presented by DCs after uptake of CMV-infected fibroblasts
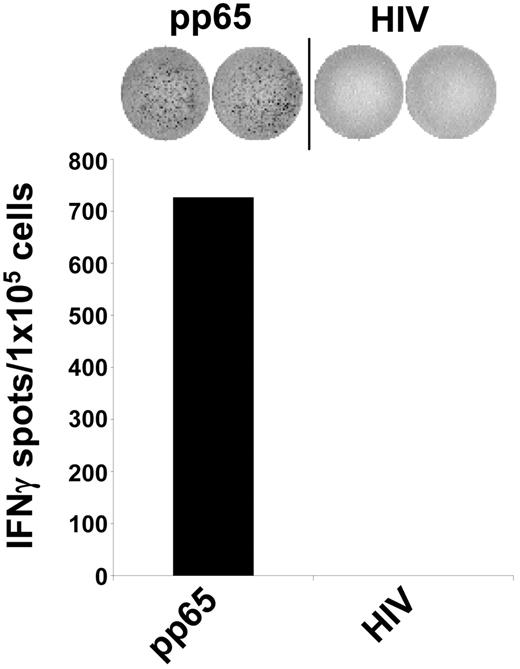
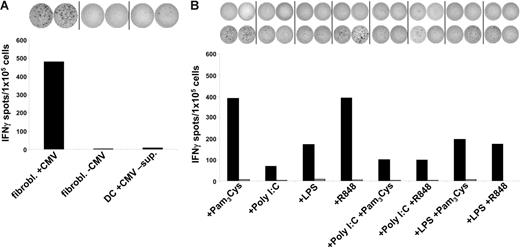
Uptake of cellular material was shown to result in cross-presentation of epitopes derived from engulfed proteins and stimulation of MHC class I–restricted cytotoxic T lymphocytes (CTLs).4–8 We therefore analyzed the impact of different DC maturation stimuli on the uptake of cells and the presentation of peptide antigens to human CTLs. To test this, we used a CTL line generated from an HLA-A2– and a CMV-positive donor by performing serial restimulations with an HLA-A2–binding peptide derived from the pp65 CMV antigen. As shown in Figure 3, this in vitro–generated CTL line specifically recognized the cognate pp65 peptide. Stimulation of T cells with autologous DCs pulsed with an irrelevant HIV peptide resulted only in background IFN-γ production, as analyzed in an ELIspot assay. In the next set of experiments, autologous DCs were incubated with irradiated HLA-A2–negative HFFs either infected with CMV or left untreated and were used as stimulators in ELIspot assays. As shown in Figure 4A, immature DCs efficiently stimulated IFN-γ secretion by CMV-specific autologous CTLs when they were cocultured overnight with CMV-infected fibroblasts. In contrast, addition of untreated fibroblasts to the DC cultures or DCs that were coincubated with supernatant derived from CMV infection of fibroblasts as a control (Figure 4A) had no effect, as was the case if phagocytosis was performed at 4°C (data not shown).
Antigen specificity of the generated CMV-CTL line. A CTL line specific for the HLA-A2 binding pp65-CMV antigen–derived peptide was generated from a CMV-positive healthy donor (HLA-A2+). The specificity of the CTLs was analyzed in IFN-γ ELIspot assays. The CTL line raised against an HLA-A2–binding pp65-derived peptide, specifically recognized autologous DCs loaded with the corresponding peptide but not an irrelevant HIV-derived peptide. The data shown are representative of 5 independent experiments.
Antigen specificity of the generated CMV-CTL line. A CTL line specific for the HLA-A2 binding pp65-CMV antigen–derived peptide was generated from a CMV-positive healthy donor (HLA-A2+). The specificity of the CTLs was analyzed in IFN-γ ELIspot assays. The CTL line raised against an HLA-A2–binding pp65-derived peptide, specifically recognized autologous DCs loaded with the corresponding peptide but not an irrelevant HIV-derived peptide. The data shown are representative of 5 independent experiments.
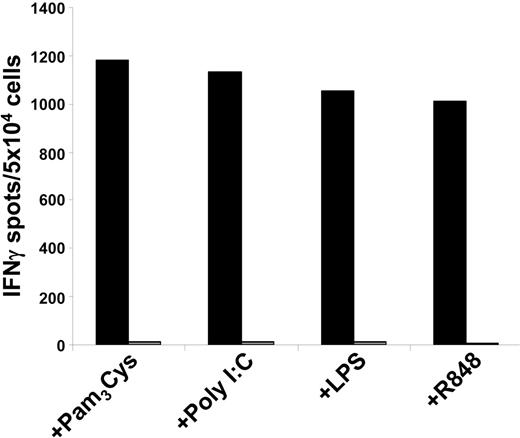
Cross-presentation of viral antigens is impaired upon TLR3 or TLR4 activation. Immature DCs or DCs matured with different TLR ligands were cocultivated for 24 hours with CMV-infected (+CMV and black columns) or uninfected (-CMV and gray columns) irradiated HLA-A2–negative fibroblasts and were subsequently used as stimulators in ELIspot experiments. Autologous HLA-A2–restricted pp65-peptide–specific CTLs were used as effectors. (A) Immature DCs cocultivated with CMV-infected fibroblasts (fibrobl+CMV) take up cellular material and subsequently process and cross-present these antigens on MHC class I molecules, whereas DCs cocultivated with uninfected fibroblasts (fibrobl-CMV) do not. Moreover, direct cocultivation of DCs with supernatant derived from CMV infection of fibroblasts (DC+CMV-sup) did not result in the presentation of viral antigens and subsequent stimulation of CTLs. (B) Cross-presentation of viral antigens on MHC class I molecules is strongly impaired by TLR3- or TLR4-activated DCs. Using combinations of TLR ligands such as Poly I:C/LPS and Pam3Cys/R848 did not further enhance the suppressive effect of the corresponding TLR3 or TLR4 single stimuli, respectively. The data shown are representative of at least 3 independent experiments.
Cross-presentation of viral antigens is impaired upon TLR3 or TLR4 activation. Immature DCs or DCs matured with different TLR ligands were cocultivated for 24 hours with CMV-infected (+CMV and black columns) or uninfected (-CMV and gray columns) irradiated HLA-A2–negative fibroblasts and were subsequently used as stimulators in ELIspot experiments. Autologous HLA-A2–restricted pp65-peptide–specific CTLs were used as effectors. (A) Immature DCs cocultivated with CMV-infected fibroblasts (fibrobl+CMV) take up cellular material and subsequently process and cross-present these antigens on MHC class I molecules, whereas DCs cocultivated with uninfected fibroblasts (fibrobl-CMV) do not. Moreover, direct cocultivation of DCs with supernatant derived from CMV infection of fibroblasts (DC+CMV-sup) did not result in the presentation of viral antigens and subsequent stimulation of CTLs. (B) Cross-presentation of viral antigens on MHC class I molecules is strongly impaired by TLR3- or TLR4-activated DCs. Using combinations of TLR ligands such as Poly I:C/LPS and Pam3Cys/R848 did not further enhance the suppressive effect of the corresponding TLR3 or TLR4 single stimuli, respectively. The data shown are representative of at least 3 independent experiments.
Cross-presentation of antigens derived from engulfed infected cells is impaired upon TLR3 and TLR4 activation
We next analyzed the ability of TLR ligands to affect cross-presentation of cellular antigens. To accomplish this, we used autologous immature and TLR ligand–matured DCs and cocultivated them with CMV-infected or -uninfected HLA-A2–negative fibroblasts. IFN-γ ELIspot experiments using HLA-A2–restricted pp65-peptide–specific CTLs revealed that, in line with results from previous experiments, activation of DCs with TLR3 or TLR4 ligand led to a reduced activation of the pp65-specific HLA-A2–restricted CTLs, whereas Pam3Cys- and R848-activated DCs presented CMV-derived antigens on MHC class I molecules to the same extent as immature DCs do (Figure 4B), as analyzed in an IFN-γ ELIspot assay. Recently, stimulation of DCs has been shown to be more effective in eliciting T-cell responses by using a combination of TLR ligands.14,15 Therefore, we analyzed the stimulatory capacity of DCs that were matured with simultaneous addition of 2 TLR ligands. Using combinations of Poly I:C and LPS with Pam3Cys and R848 did not lead to substantial changes in CMV-specific CTL activation in comparison to the TLR3 or TLR4 single stimuli, respectively. Similar results were obtained by using these TLR ligand combinations in phagocytosis experiments with apoptotic HEK-293 cells (data not shown).
Differential ability to stimulate antigen-specific CTLs by TLR ligand–activated DCs is not due to a variation in MHC class I surface expression
The observed impairment of antigen cross-presentation of TLR3- or TLR4-activated DCs was not due to a difference in MHC class I surface expression in TLR-activated DCs, as loading of these cells with the CMV pp65 HLA-A2–binding peptide led to comparable activation of CTLs in ELIspot experiments (Figure 5). Furthermore, phenotypic analysis (Figure 1) of the TLR-activated DCs showed that the Poly I:C- and LPS-matured DCs expressed even more MHC class I molecules on their surfaces. Moreover, analysis on the activation state of the different DC populations by staining on the activation marker CD83 and MHC class II revealed that there was no difference, as the expression levels of these molecules on the cell surface were approximately the same.
Exogenous loading of DCs with an HLA-A2–specific peptide results in equal stimulation of CTLs. TLR-stimulated DCs were exogenously pulsed with a pp65-derived (black columns) or HIV-derived (gray columns) HLA-A2–binding peptide, respectively, and were used as stimulators in IFN-γ ELIspot assays. Autologous HLA-A2–restricted pp65-peptide–specific CTLs were used as effectors. The data shown are representative of 5 independent experiments.
Exogenous loading of DCs with an HLA-A2–specific peptide results in equal stimulation of CTLs. TLR-stimulated DCs were exogenously pulsed with a pp65-derived (black columns) or HIV-derived (gray columns) HLA-A2–binding peptide, respectively, and were used as stimulators in IFN-γ ELIspot assays. Autologous HLA-A2–restricted pp65-peptide–specific CTLs were used as effectors. The data shown are representative of 5 independent experiments.
Discussion
The life cycle of DCs is mainly characterized by antigen uptake in the periphery, followed by activation upon interaction with pathogens, inflammatory signals, or cytokines, migration to regional lymph nodes, and presentation of antigen-derived peptides to T lymphocytes.16 The present study demonstrates that antigen uptake and subsequent cross-presentation of antigen-derived T-cell epitopes by DCs are differentially regulated upon maturation of DCs with distinct TLR ligands. We show that activation of DCs with Poly I:C (TLR3 ligand) or LPS (TLR4 ligand) dramatically reduced the uptake of apoptotic cells compared with immature DCs, as described previously.17,18 But surprisingly, activation of DCs with Pam3Cys (TLR2 ligand) or R848 (TLR7 ligand) had no impact on the ability to ingest cellular material, as assessed in cocultivation experiments using membrane-dye–labeled cells. This might be due to the different signaling pathways engaged upon activation of the specific TLRs.9 TLR2 and TLR7 signaling are MyD88 dependent, which leads to the activation and nuclear localization of NFκB. This is also true for TLR4-mediated signal transduction, but ligand-binding by this receptor, likewise stimulation of TLR3, could also induce activation of a MyD88-independent pathway that leads to activation of the transcription factor IRF-3. This molecule is known to be involved in IFN-stimulated gene transcription, which functions as a direct response to viral infection and is potently involved in inhibiting viral replication.19
Moreover, TLR activation also seems to affect the uptake of CMV-infected fibroblasts, leading to cross-presentation of CMV-derived peptides on MHC class I molecules and activation of CMV pp65-specific CD8+ T lymphocytes. In line with the phagocytosis experiments using apoptotic HEK-293 cells, stimulation of DCs with TLR3 or TLR4 ligands but not with TLR2 or TLR7 ligands resulted in a reduced activation of pp65-specific HLA-A2–restricted CTLs. This difference in stimulation capability was not dependent on differential regulation of MHC class I surface expression, as TLR-stimulated and peptide-loaded DCs showed comparable activation capacity of CTLs. Presentation of CMV-specific peptides was not due to secondary infection of DCs in the coculture because the laboratory strand of CMV (AD169) used in our experiments shows a fibroblast-specific cell tropism and does not infect DCs.20,21 These results demonstrate that, depending on the stimuli provided from the environment to DCs, a viral infection might result in the induction of a protective T-cell–mediated immunity or an inhibition of immune responses against the pathogen, resulting in its escape.
The observed down-regulation of antigen uptake and cross-presentation by TLR3- or TLR4-stimulated DCs were also described in recent publications.17,18,22 Wilson et al could show in mice that cross-priming of antigens and antiviral responses was greatly impaired upon systemic exposure of animals to Poly I:C, LPS, or CpG. Similar results could be obtained by Gil-Torregrosa et al17 and West et al,22 who used LPS to stimulate DCs. Furthermore, they observed an enhanced ability to take up antigens during the first few hours after giving the stimulus, followed by an absolute down-regulation upon complete maturation of the cells. In contrast, there are also reports showing differing observations on cross-presenting abilities upon activation with TLRs.23,24 Most of the recent experiments have been done in mice.17,18,22–24 Due to different functional abilities of human and mouse DC subsets and diverse expression of TLRs, the direct transfer of these results to the human system has to be elucidated. Our results obtained with human DCs are of special interest, as TLR ligands are currently used as adjuvants in vaccination studies. Depending on the antigen used in clinical trials, a different set of activation stimuli has to be applied. In respect to this, vaccination strategies that use antigens that do not require further processing and cross-presentation by APC, like peptides in antitumor approaches, can take advantage of DC activation by TLR3 or TLR4. Such matured cells secrete huge amounts of proinflammatory cytokines such as IL-6 and IL-12. Furthermore, it has been shown that IL-12 production could be increased up to 50-fold by addition of a combination of one of these stimuli with TLR7/8 ligands, than by the addition of a single agonist.14,15 However, antigens that need to be cross-presented, such as soluble proteins, immune complexes, or cellular-associated antigens, should be used with ligands specific for TLR2 and/or TLR7/8 as adjuvants for DC maturation.
In summary, the data reported here show that clearing of apoptotic cells and cross-presentation of cellular antigens on MHC class I molecules to specific CTLs is differentially regulated upon activation of DCs with different TLR ligands. This may have an impact on the design and development of vaccination studies. Furthermore, it may contribute additional insight into the interaction of pathogens with antigen-presenting cells and the subsequent induction of pathogen-directed immune responses, which might result in protection or escape from the immune system.
Authorship
Contribution: M.M.W. performed research, analyzed data, and wrote the paper; F.G. and P.B. designed the research; D.W. and A.B. performed research and analyzed data; C.S. contributed analytical tools.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Brossart, University of Tübingen, Department of Internal Medicine II, Oncology, Hematology, Immunology, Rheumatology and Pulmology, Otfried-Müller-Str 10, 72076 Tübingen, Germany; e-mail: peter.brossart@med.uni-tuebingen.de.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank K. Laib-Sampaio (University of Tübingen, Institute of Medical Virology, Tübingen, Germany) for help with the CMV experiments, B. Fehrenbacher and M. Schaller (University of Tübingen, Department of Dermatology, Tübingen, Germany) for confocal laser scanning microscopy, and B. Drotlef, S. Stephan, and S. Daecke for excellent technical assistance. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 685).