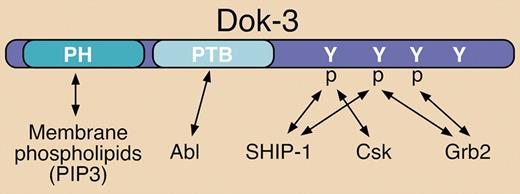
The Dok family of cytosolic adaptor proteins consists of 7 members. Dok-1, Dok-2, and Dok-3 are closely related and function as negative signaling regulators. They are predominantly expressed in hematopoietic cells and share similar pleckstrin homology (PH) and phosphotyrosine binding (PTB) domain structural elements (see figure). Dok-3 (also called DOKL) is expressed in B lymphocytes, mast cells, and myeloid cells.1,2
Structural elements and known binding partners of Dok-3. Dok-3 interacts with various signaling proteins via a PTB domain and several C-terminal phosphorylated (p) tyrosine (Y) residues. Known interactions are indicated by double-sided arrows. The pleckstrin homology (PH) domain can interact with phosphatidylinositol 3,4,5-trisphosphate (PIP3) at the plasma membrane.
Structural elements and known binding partners of Dok-3. Dok-3 interacts with various signaling proteins via a PTB domain and several C-terminal phosphorylated (p) tyrosine (Y) residues. Known interactions are indicated by double-sided arrows. The pleckstrin homology (PH) domain can interact with phosphatidylinositol 3,4,5-trisphosphate (PIP3) at the plasma membrane.
In B cells, Dok-3 has been shown to be rapidly tyrosine phosphorylated upon antigen receptor (BCR) engagement, which promotes recruitment of SH2 domain-containing 5′-inositol phosphatase-1 (SHIP-1) to mediate negative signaling (see figure).2,3 Previous reports demonstrated that overexpression of Dok-3 in B-cell lines can suppress BCR-stimulated cytokine production, NFAT activation, and JNK activation, while calcium mobilization and activation of other MAP kinases were unaffected.2,3 In contrast, overexpression of a mutant form of Dok-3 lacking C-terminal tyrosine residues required for SHIP-1 association resulted in enhancement of the same responses upon BCR engagement.2 Csk can also be recruited to tyrosine-phosphorylated Dok-3 (see figure), but this inhibitory protein tyrosine kinase reportedly is not responsible for mediating the negative signaling functions described.2,–4
More recent studies have established that tyrosine-phosphorylated Dok-3 can also recruit the Grb2 adaptor (see figure).4,5 Evidence for 2 negative impacts from this interaction have been reported: 1) The Dok-3/Grb2 complex is directly involved in diminishing calcium signaling in response to BCR engagement5 ; and 2) Dok-3 sequesters Grb2 away from the GTP exchange factor (GEF) Sos, thereby preventing efficient activation of the Ras/ERK cascade.4 Related to the latter report, overexpression of wild-type Dok-3 has been shown to inhibit v-Abl–induced ERK activation and to blunt the transforming capacity of v-Abl.1
In this issue of Blood, Ng and colleagues present the first characterization of B cells from Dok-3–deficient mice. These mice were found to have normal B-cell development, but elevated levels of serum immunoglobulin M (IgM) and potentiated antibody responses to T-independent antigens. Furthermore, the B cells from these mice proliferated significantly more robustly and demonstrated enhanced calcium signaling in response to BCR crosslinking. In addition, BCR-stimulated activation of NFκB, JNK, and p38 were potentiated in Dok-3–deficient B cells. In contrast to previous studies in cell lines, however, ERK activation was not altered in Dok-3–deficient B cells. Further work is required to establish the basis for the seemingly contradictory impacts on ERK. The authors have also provided evidence that SHIP-1 recruitment to the plasma membrane is normal in Dok-3–deficient B cells, but tyrosine phosphorylation of the enzyme is diminished. This intriguing result suggests that Dok-3 serves as a platform to bring a protein tyrosine kinase in contact with SHIP-1, which is important for activating catalytic function of the inositol phosphatase.
As an extension of the previous studies in cell lines, the studies of Ng and colleagues significantly improve our understanding of the role of Dok-3 in normal B-cell biology. Furthermore, in contrast to Dok-1, which was previously shown to elicit negative regulation of BCR signaling through FcγRIIb, the current work establishes a more direct role for Dok-3 in attenuating BCR signaling responses. Thus, while both Dok-1 and Dok-3 negatively regulate the BCR, they function in a non-redundant manner.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■