Abstract
Apoptosis of CD4+ T cells and TH2 polarization are hallmarks of sepsis-induced immunoparalysis. In this study, we characterized sepsis-induced adaptive immune dysfunction and examined whether improving T-cell effector function can improve outcome to sepsis. We found that septic mice produced less antigen-specific T-cell–dependent IgM and IgG2a antibodies than sham-treated mice. As early as 24 hours after sepsis, CD4+ T cells proliferated poorly to T-cell receptor stimulation, despite normal responses to phorbol myristate acetate and ionomycin, and possessed decreased levels of CD3ζ. Five days following immunization, CD4+ T cells from septic mice displayed decreased antigen-specific proliferation and production of IL-2 and IFN-γ but showed no difference in IL-4, IL-5, or IL-10 production. Treatment of mice with anti-GITR agonistic antibody restored CD4+ T-cell proliferation, increased TH1 and TH2 cytokine production, partially prevented CD3ζ down-regulation, decreased bacteremia, and increased sepsis survival. Depletion of CD4+ T cells but not CD25+ regulatory T cells eliminated the survival benefit of anti-GITR treatment. These results indicate that CD4+ T-cell dysfunction is a key component of sepsis and that improving T-cell effector function may be protective against sepsis-associated immunoparalysis.
Introduction
Despite numerous studies showing that prevention of lymphocyte apoptosis improves survival in animal models of sepsis,1-4 only a few studies using cytokine/anticytokine therapies have examined whether improving leukocyte function directly may improve outcome during sepsis.5-7 In a previous study, we found that despite increased regulatory T-cell (Treg) activity in sepsis, depletion of Tregs, and even depletion of all CD4+ T cells, did not affect mortality in sepsis.8 However, even in the absence of Tregs, T-effector cells from septic mice did not proliferate as well as T-effector cells from sham-treated mice. These findings suggest that the endogenous CD4+ population does not play a major role in outcome during sepsis, likely due to sepsis-induced dysfunction in CD4+ T cells and enhanced apoptosis. In contrast, augmentation of CD4+ T-cell effector responses, either by inhibition of apoptosis or by direct stimulation, may be necessary to overcome this dysfunction.
Glucocorticoid-induced tumor necrosis factor (TNF) receptor family–related gene (GITR) is a member of the TNF receptor superfamily that is expressed normally at high levels on CD4+CD25+ Tregs. It is expressed at low levels on resting CD4+CD25− and CD8+ CD25− T-effector cells but increases upon activation. Initially, GITR was found to abrogate Treg-mediated suppression,9 but it was also found to act as a costimulatory molecule on effector T cells.10,11
We hypothesized that stimulating CD4+ effector T-cell function might be an appropriate therapeutic target during sepsis. We analyzed lymphocyte function in sepsis and determined whether anti-GITR treatment could prevent the adaptive immune dysfunction and improve survival in sepsis. We found that T-cell–dependent IgM and IgG2a antibody production is reduced in septic mice. Furthermore, we find that CD4+ T cells isolated from septic mice exhibit decreased CD3ζ chain expression, proliferate poorly to antigen nonspecific and antigen-specific stimulation, and produce less IL-2 and IFN-γ than CD4+ T cells obtained from sham-treated mice. We demonstrate that treatment with a GITR agonistic antibody increases T-cell–dependent antibody class switching to both IgG2a and IgG1, increases CD3ζ expression, restores proliferation, and increases both TH1 and TH2 cytokine production in T cells from septic mice, leading to an improvement in survival. This survival improvement was abrogated in mice depleted of CD4+ T cells but not CD4+CD25+ T-regulatory cells. These data indicate that augmenting T-cell function may be an appropriate target in sepsis.
Materials and methods
Mice
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Florida College of Medicine before their initiation. All mice were maintained on standard rodent food and water ad libitum. Specific pathogen-free 6-week-old C57Bl/6 and Balb/cJ female mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and were used between 8 and 12 weeks of age. T-cell transgenic C.Cg-Tg(DO11.10)10Dlo/J (DO11.10) breeding pairs were purchased from the Jackson Laboratory and maintained in specific pathogen-free conditions at a breeding colony at the University of Florida Health Science Center.
Reagents
NP (4-hydroxy-3-nitrophenyl) acetyl-KLH (keyhole limpet hemocyanin) and NP–bovine serum albumin (BSA) were purchased from Biosearch Technologies (Novato, CA). An antigenic peptide corresponding to amino acids 323–339 of chicken ovalbumin (Ova323–339) was purchased from Genscript (Piscataway, NJ). Phorbol-12-myristate-13-acetate and ionomycin were purchased from EMD Biosciences (Darmstadt, Germany). Rat anti–mouse GITR agonistic (DTA-1), anti-CD4 (GK1.5), and anti-CD25 (PC61) purified antibodies were purchased from BioExpress (West Lebanon, NH). Rat anti–human IL-4 used as an IgG2b isotype control antibody for in vivo studies was obtained from Schering Plough Biopharma (Palo Alto, CA). CD4, CD90 were purchased from Miltenyi Biotech (Auburn, CA).
CLP
For induction of polymicrobial sepsis, mice were subjected to cecal ligation and puncture (CLP) as previously described,8 with a few modifications. In brief, a laparotomy was performed, the cecum was isolated, and approximately 0.75 cm to 1 cm of cecum was ligated below the ileocecal valve and punctured through and through with a 23-gauge needle. Sham operation was performed by isolating the cecum without ligation or puncture. When indicated, animals were given either an intraperitoneal injection of 300 μg of anti-GITR or isotype control antibody 30 minutes before surgery. Also, when indicated, mice were immunized at the time of surgery with nitrophenyl–keyhole limpet hemocyanin (NP-KLH; 100 μg) or 100 μg Ova323-339 mixed with alum (100 μL), dissolved in phosphate-buffered saline (PBS). In 2 experiments, depletion of CD4+ T cells or CD25+ T cells was achieved by intraperitoneal injection of either anti-CD4 antibody (GK1.5 hybridoma) or anti-CD25 antibody (PC61 hybridoma; 500 μg) 3 days prior to surgery and again at the time of anti-GITR treatment (250 μg). Depletion was confirmed by flow cytometry and lasted up to 8 days (Scumpia et al8 and data not shown). Depending on the experiment, mice were either killed at 24 hours or 5 days after surgery to harvest splenocytes, bone marrow, and/or lymph nodes or animals were observed for up to 10 days to determine survival or humoral antibody responses.
Flow cytometry
Spleens, peripheral lymph nodes (inguinal and axillary), mesenteric lymph nodes, and/or bone marrow were harvested after CLP or sham surgery, and single-cell suspensions were created by passing the cells through a 70-μm pore-size cell strainer (Falcon, Heidelberg, Germany). Erythrocytes were lysed with an ammonium chloride lysis solution. After washing twice with PBS without phenol red, calcium, and magnesium (Cellgro; Mediatech, Herndon, VA), cells were resuspended in PBS containing 0.2% anti-CD16/32 for Fc blocking and then stained. All antibodies were purchased from BD Pharmingen (San Jose, CA) unless otherwise stated. Antibodies used include anti-CD4 (RM4.4, conjugated to FITC; or GK1.5 conjugated to allophycocyanin), anti-CD69 (H1.2F3, conjugated to PE), anti-CD28 (37.51, conjugated to PE; eBioscience, San Diego, CA), anti–CTLA-4 (UC10–4B9, conjugated to PE), anti-GITR (DTA-1, conjugated to allophycocyanin), antimouse DO11.10 TCR (KJI-26, conjugated to allophycocyanin), anti-CD3ϵ (500A2, conjugated to Pacific Blue), anti-CD3ζ (H146–968, conjugated to FITC; Cedarlane Labs, ON, Canada; or 6B10.2, conjugated to PE; Santa Cruz Biotechnologies, Santa Cruz, CA). For intracellular staining for CD3ζ, extracellular staining was performed followed by intracellular staining using commercially available fixation and permeabilization solutions (eBioscience) and their recommended directions. Samples were acquired and analyzed on either a FACSCalibur or an LSRII flow cytometer (BD Biosciences, San Jose, CA). At least 104 live (7-aminoactinomycin D [7AAD−] or Sytox Blue−) cells were analyzed, except for intracellular staining where 4 × 104 total events were analyzed.
Immunization with NP-KLH and NP-specific ELISA
T-cell–dependent humoral immune responses were quantified using an NP-KLH immunization model as previously described by Hurov et al.12 Briefly, tail vein bleeds were completed on naive C57Bl/6 mice prior to no (control), sham, or cecal ligation and puncture treatment. When indicated, animals were given either an injection of anti-GITR or isotype control antibody 30 minutes before CLP. At the time of surgery, mice were immunized with 100 μg of NP-KLH in alum subcutaneously. Ten days after immunization, mice were killed and bled by cardiac puncture. Titers of NP-specific antibodies from serum samples were determined by enzyme-linked immunosorbent assay (ELISA). Immulon 4HBX 96-well plates (Dynex Technologies, Ashford, United Kingdom) were coated with 1 mg of NP–bovine serum albumin (Biosearch Technologies) per mL. Serum samples were bound to the plates in dilutions from 1:100 to 1:5 × 107. NP-specific antibodies were then bound to biotin-conjugated goat anti–mouse Ig isotype antibodies, anti-IgM, anti-IgG1, and anti-IgG2a (CalTag, Burlingame, CA). Streptavidin-conjugated horseradish peroxidase was incorporated to detect the biotin-Ig with 2,2′-azino-di(3-ethylbenzthiazolinesulfonate [ABTS]) substrate. Titers of Ig from individual mice were obtained by determining the dilution at which serum samples gave optical density readings at 414 nm of 0.2 units above background. No significant levels of NP-specific IgG existed prior to initiation of either sham or cecal ligation and puncture procedure.
Cell purification
All magnetic beads were obtained from Miltenyi Biotec. Splenocytes were collected as described above. The CD4+ T-cell population from septic or sham mice was purified by positive selection using CD4 (L3T4) microbeads (> 95% purity). To deplete CD25+ cells from the total CD4+ population, high-speed cell sorting with CD3-FITC and CD25-PE on a FACSVantage (BD Pharmingen) was performed to obtain CD3+CD4+CD25− cells (Teffs) at greater than 98% purity. Antigen-presenting cells (APCs) were obtained from control mice by either incubating splenocytes with CD90 microbeads then running them through LD columns (∼97% T-cell depleted) using the manufacturer's instructions or using the CD4− fraction from the L3T4 microbeads. These cells were then irradiated (30 Gy). All cell counts were performed on a hemocytometer using trypan blue to exclude dead cells from the counts before culture.
Proliferation assay
After purification and washing, a proliferation assay was developed to test the capacity of total CD4+ T cells or CD4+CD25− T-effector cells to proliferate in response to a polyclonal stimulus or to a specific peptide. T cells (2.5 × 104 cells/well) were cultured with irradiated antigen-presenting cells (2.5 × 105 cells/well) in at least triplicate. Soluble anti-CD3 (2.5 μg/mL) and 1 μg anti-CD28 provided the polyclonal stimulus for T-cell receptor stimulation, 50 ng/mL PMA and 1 μM ionomycin provided the non–T-cell receptor–mediated stimulation, whereas 5 μg/mL Ova323-339 provided the antigen-specific stimulus for proliferation over a 48- to 72-hour culture period in a total volume of 200 μL of complete RPMI medium. Media were harvested from each well for cytokine determination after 30 hours. Then, 1 μCi (0.037 MBq) 3H-thymidine (Amersham Biosciences, Piscataway, NJ) was added for the final 14 to 18 hours of culture to assess proliferation. In preliminary studies, a regulatory T cell–to–effector T-cell ratio of at least 1:2 (using Tregs from either sham-treated or septic mice) was necessary to develop suppression of T-effector cell responses (data not shown), so depletion of CD25+ T cells (∼7%-15% of all CD4 cells) from the total CD4 pool did not significantly affect T-cell proliferation of either sham-treated or septic mice. For this reason, the first proliferation experiment contains CD25-depleted CD4+ T cells and the remaining experiments use total CD4+ T cells in the proliferation studies.
In vivo expansion of antigen-specific T cells
CD4+ T cells (5 × 106) isolated by positive selection from the spleens of DO11.10 Tg mice were transferred via tail vein into Balb/c mice 3 days before sham or induction of a CLP. Immediately following surgery, mice were immunized subcutaneously with Ova peptide (100 μg) in alum and 5 days later were killed, and peripheral lymph nodes were harvested for flow cytometry to determine the expansion of KJI-26+CD4+ T cells in the lymph nodes.
Determination of bacteremia
Blood bacteremia was determined by culturing 10 μL of whole blood diluted with 90 μL of sterile physiologic saline. This solution was then plated in serial dilutions on sheep's blood agar plates (Fisher Scientific, Pittsburgh, PA) at 37°C in 5% CO2. Plates were counted after 48 hours of culture.
Multiplex cytokine analysis
Assessments of cytokine profiles from the proliferation assay were performed using a commercially available multiplexed kit (Beadlyte Mouse Multi-Cytokine Detection System 2; Upstate Biotechnology, Waltham, MA) and the Luminex (100) LabMAP System (Austin, TX). Simultaneous measurement of 10 cytokines was performed: specifically IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p70), tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), and granulocyte monocyte–colony-stimulating factor (GM-CSF). All assays were performed according to the manufacturer's protocols. Samples from at least 3 replicate wells were then diluted 1:2 in tissue culture medium before analysis. Cytokine concentrations were determined using SOFTmax PRO version 4.3 software (Molecular Devices, Sunnyvale, CA) with 4-parameter data analysis.
Statistics
Differences in survival were determined by the Fisher exact test. Continuous variables were first tested for normality and equality of variances. Differences among groups were evaluated by Student t test or 1-way analysis of variance (ANOVA), when comparing more than 2 groups. If differences were found between multiple groups, Fisher least significant difference (LSD) tests were performed comparing groups of interest. Significance was determined at the 95% confidence level.
Results
Sepsis decreases T-cell–dependent antibody responses
In a seminal study by MacLean et al,13 it was observed that septic patients and trauma patients that progressed to sepsis displayed an inability to develop delayed-type hypersensitivity responses. We initially used a standard system whereby we could test whether an adaptive T-cell–dependent immune response can develop in septic mice by immunizing subcutaneously with the T-cell–dependent antigen NP-KLH mixed with alum and examining antigen-specific antibody production. Not surprisingly, septic mice displayed a decreased ability to produce NP-specific IgM and IgG2a antibodies, whereas IgG1 antibody responses (the predominant isotype of IgG antibodies formed when alum is used as an adjuvant) were largely intact (Figure 1A-C).
Anti-GITR treatment improves T-cell–dependent antibody responses in septic mice. C57Bl/6 mice underwent a sham procedure or cecal ligation and puncture (CLP) surgery and were immunized subcutaneously with the T-cell–dependent antigen NP-KLH and alum. Ten days later, serum (A) IgM, (B) IgG2a, and (C) IgG1 anti-NP antibody responses were measured by ELISA and compared with baseline samples. In another experiment, C57Bl/6 mice were given an intraperitoneal injection of 300 μg control antibody (Sham and CLP groups) or anti-GITR antibody and underwent cecal ligation and puncture or sham treatment 30 minutes later. Immediately following, mice were immunized with NP-KLH and alum. Ten days later, mice were bled and anti-NP–specific IgM (A), IgG2a (B), and IgG1 (C) were measured from the serum. P values indicate the difference between groups after immunization by Student t test (A-C) or post hoc analysis using Fisher LSD method (D-F).
Anti-GITR treatment improves T-cell–dependent antibody responses in septic mice. C57Bl/6 mice underwent a sham procedure or cecal ligation and puncture (CLP) surgery and were immunized subcutaneously with the T-cell–dependent antigen NP-KLH and alum. Ten days later, serum (A) IgM, (B) IgG2a, and (C) IgG1 anti-NP antibody responses were measured by ELISA and compared with baseline samples. In another experiment, C57Bl/6 mice were given an intraperitoneal injection of 300 μg control antibody (Sham and CLP groups) or anti-GITR antibody and underwent cecal ligation and puncture or sham treatment 30 minutes later. Immediately following, mice were immunized with NP-KLH and alum. Ten days later, mice were bled and anti-NP–specific IgM (A), IgG2a (B), and IgG1 (C) were measured from the serum. P values indicate the difference between groups after immunization by Student t test (A-C) or post hoc analysis using Fisher LSD method (D-F).
Anti-GITR treatment improves antibody responses in sepsis
Signaling through GITR not only neutralizes the suppressive effect of Tregs but also augments activation, proliferation, and cytokine production of effector T cells.14 We recently found that GITRhigh-expressing T cells increase in septic mice compared with sham mice.8 In this study, we examined whether increased GITR signaling on T cells may augment adaptive immune function and prevent the immune suppression that occurs in sepsis. C57Bl/6 mice were injected with 300 μg of anti-GITR agonistic antibody (DTA-1) or a control antibody intraperitoneally 30 minutes before the initiation of CLP, sham surgery, or no treatment. In mice receiving no surgery, the administration of anti-GITR antibody prior to immunization with NP-KLH and alum resulted in an increase in serum anti-NP–specific IgM, IgG1, and IgG2a titers (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Next, we assessed whether anti-GITR treatment can also improve humoral immune responses in sepsis. We found that anti-GITR treatment did not improve anti-NP IgM production (Figure 1D) but caused a dramatic improvement in IgG2a (Figure 1E) and increased IgG1 (Figure 1F), indicating that anti-GITR treatment improves class-switching to both TH1- and TH2-type immune responses in septic mice.
Impaired CD4+ T-cell function in early sepsis is associated with decreased CD3ζ expression
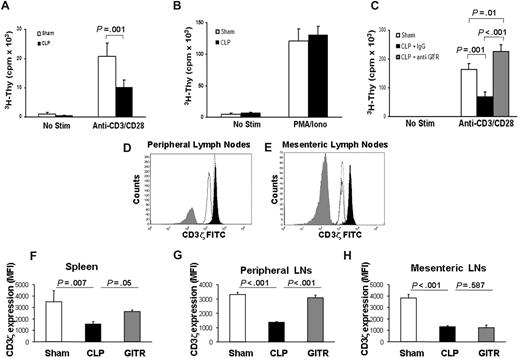
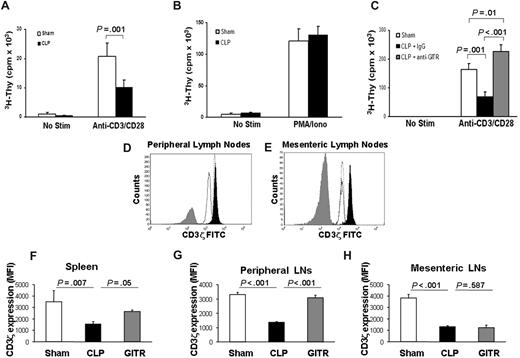
To elucidate the effects of sepsis on TH cells, we examined the expression of costimulatory (CD28), inhibitory (CTLA-4), and activation (CD69, GITR) molecules by flow cytometry. Sepsis caused a decrease in the expression of cell-surface CD28 (Figure S1A), whereas it increased the cell-surface expression of CTLA-4, CD69, and GITR on the surface of CD3+CD4+ T cells (Figure S1B-D). These results were similar to those of Unsinger et al15 who also found that CD4+ T cells in spleens of septic mice display increased CD69 expression. However, despite increased activation marker expression, TH cells from septic mice proliferated poorly in response to polyclonal anti-CD3/CD28 stimulation 24 hours after sepsis (Figure 2A). When stimulated with PMA and ionomycin, however, their proliferative response was largely intact (Figure 2B). The fact that antigen-presenting cells from naive mice were cultured with T cells from both sham and septic mice highlights the fact that T cells from septic mice even when removed from the septic environment remain dysfunctional.
T cells from septic mice proliferate poorly to TCR stimulation and display decreased CD3ζ expression. Twenty-four hours after cecal ligation and puncture (CLP) or sham treatment, CD25-depleted CD4+ T-effector cells were harvested as described in “Proliferation assay.” T-effector cells (2.5 × 104) were cultured for 72 hours with anti-CD3 (2.5 μg/mL) and anti-CD28 (1 μg/mL; A) or PMA and ionomycin (B). 3H-thymidine was added during the last 14 to 18 hours of culture and incorporation was measured using a liquid scintillation counter. (C) Mice were treated as in panel A, except prior to CLP, mice were given an intraperitoneal injection of anti-GITR or control antibody (300 μg). Mice were given 300 μg anti-GITR or control antibody at the time of CLP surgery. Twenty-four hours after CLP or sham surgery, cells were stained extracellularly for flow cytometry using anti-CD3ϵ Pacific Blue and anti-CD4 allophycocyanin. Cells were then fixed and permeabilized as described in “Flow cytometry” and stained for anti-CD3ζ FITC. (D,E) Histogram overlays showing CD3ζ from CD4+ T cells of 1 sham mouse (filled black) versus 1 control antibody–treated CLP mouse (solid black line, no fill) versus 1 anti-GITR–treated CLP mouse (dotted line, no fill) and an isotype control antibody (filled gray) from peripheral lymph nodes (D) or mesenteric lymph nodes (E). The calculated mean fluorescent intensity (MFI) from (F) spleen, (G) peripheral lymph nodes, and (H) mesenteric lymph nodes from n = 3 mice per group. Histogram overlays were made using FCS Express software version 3 (De Novo, Los Angeles, CA). P values are indicative of the difference between groups using Student t test (A) or post hoc analysis using the Fisher LSD method. All error bars indicate SD.
T cells from septic mice proliferate poorly to TCR stimulation and display decreased CD3ζ expression. Twenty-four hours after cecal ligation and puncture (CLP) or sham treatment, CD25-depleted CD4+ T-effector cells were harvested as described in “Proliferation assay.” T-effector cells (2.5 × 104) were cultured for 72 hours with anti-CD3 (2.5 μg/mL) and anti-CD28 (1 μg/mL; A) or PMA and ionomycin (B). 3H-thymidine was added during the last 14 to 18 hours of culture and incorporation was measured using a liquid scintillation counter. (C) Mice were treated as in panel A, except prior to CLP, mice were given an intraperitoneal injection of anti-GITR or control antibody (300 μg). Mice were given 300 μg anti-GITR or control antibody at the time of CLP surgery. Twenty-four hours after CLP or sham surgery, cells were stained extracellularly for flow cytometry using anti-CD3ϵ Pacific Blue and anti-CD4 allophycocyanin. Cells were then fixed and permeabilized as described in “Flow cytometry” and stained for anti-CD3ζ FITC. (D,E) Histogram overlays showing CD3ζ from CD4+ T cells of 1 sham mouse (filled black) versus 1 control antibody–treated CLP mouse (solid black line, no fill) versus 1 anti-GITR–treated CLP mouse (dotted line, no fill) and an isotype control antibody (filled gray) from peripheral lymph nodes (D) or mesenteric lymph nodes (E). The calculated mean fluorescent intensity (MFI) from (F) spleen, (G) peripheral lymph nodes, and (H) mesenteric lymph nodes from n = 3 mice per group. Histogram overlays were made using FCS Express software version 3 (De Novo, Los Angeles, CA). P values are indicative of the difference between groups using Student t test (A) or post hoc analysis using the Fisher LSD method. All error bars indicate SD.
Since T-cell–dependent antibody production was improved in septic mice treated with anti-GITR, we next assessed the effects of anti-GITR treatment on early T-cell proliferation. Interestingly, 24 hours after sepsis, there was a marked improvement of septic T cells to proliferate to polyclonal stimulus when the mice were treated with an anti-GITR antibody but not the control antibody (Figure 2C). In fact, early CD4+ proliferation was improved over sham levels (Figure 2C).
Since T cells from septic and sham-treated mice proliferated to similar levels in response to PMA/ionomycin stimulation, this may suggest that downstream signaling components from the T-cell receptor (TCR) were still functional. We therefore examined whether expression of components of the TCR complex were altered in acute sepsis. CD3ζ chain is the limiting factor in TCR assembly,15 and down-regulation of the CD3ζ chain has been implicated in T-cell dysfunction and anergy in many chronic inflammatory diseases.16-18 We assessed whether acute sepsis could cause down-regulation of CD3ζ. Indeed, we found using 2 different clones of anti-CD3ζ antibody that 24 hours after sepsis there was a marked decrease in CD3ζ expression, with no decrease in CD3ϵ expression in spleen or peripheral or mesenteric lymph nodes (Figure 2D-H; and data not shown).
Since anti-GITR treatment was able to improve T-cell functions, we examined anti-GITR effects on T-cell receptor expression. Anti-GITR treatment restored CD3ζ chain expression on CD4+ T cells in the spleen (∼50%) and peripheral lymph nodes (∼90%) but not in mesenteric lymph nodes (Figure 2D-H). These data suggest that anti-GITR reversal of T-cell hyporesponsiveness is associated with at least a partial restoration of CD3ζ chain expression in the mice treated with anti-GITR.
Antigen-specific TH-cell proliferation and cytokine production are inhibited in sepsis and corrected by anti-GITR treatment
Thus far, these studies revealed that polyclonal T-cell activation was reduced during sepsis. Next we assessed whether antigen-specific CD4+ T-cell responses were similarly decreased in sepsis using DO11.10 TCR transgenic mice. Five days after surgery and subcutaneous immunization, septic DO11.10 CD4+ T cells from draining lymph nodes proliferated less (Figure 3A) and produced profoundly less IL-2 and IFN-γ (Figure 3B,C) than cells from sham-treated mice upon ex vivo restimulation with specific Ova(323-339) peptide. Interestingly, no difference was found in the levels of IL-4, IL-5, IL-10, TNF-α, IL-1β, and GM-CSF between stimulated T cells from sham-treated and septic mice (Figure 3D,E; and data not shown). Taken together with the early septic T-cell proliferative dysfunction, these data indicate that both antigen-specific and nonspecific CD4+ T-cell dysfunction occur in sepsis.
Sepsis-induced antigen-specific CD4+ T-cell dysfunction is corrected by anti-GITR treatment. DO11.10 mice were administered 300 μg anti-GITR or control antibody and were immunized 30 minutes later with Ova323-339 in alum at the time of cecal ligation and puncture or sham surgery. Five days later, lymph node cells were harvested and 2.5 × 104 CD4+ cells were cultured with 2.5 × 105 irradiated APCs with Ova peptide for 72 hours. (A) Proliferation of CD4+ T cells was measured as the incorporation of 3H-thymidine during the last 18 hours of culture. Media was harvested at the time of 3H-thymidine addition to assess cytokine production. (B) IL-2, (C) IFN-γ, (D) IL-4, and (E) IL-10 concentrations were assessed by Luminex multiplex analysis. (F) Representative examples of flow plots demonstrating the expansion of DO11.10 T cells (KJI-26+CD4+) from lymph nodes of Balb/c mice that were injected intravenously with 5 × 106 DO11.10 CD4+ T cells 3 days before sham (left), CLP with control antibody (middle), or CLP with anti-GITR antibody (right) treatment at the time of immunization with Ova323-337 peptide in alum. (G) The calculated percentage of living (Sytox Blue−), nondebris DO11.10 T cells (KJI26+CD4+) expanded in the peripheral lymph nodes of the Balb/c mice. (H) The calculated total number of live (Sytox Blue−), nondebris DO11.10 T cells using cell counts obtained with a hemacytometer. P values indicate differences between groups after post hoc analysis using the Fisher LSD method.
Sepsis-induced antigen-specific CD4+ T-cell dysfunction is corrected by anti-GITR treatment. DO11.10 mice were administered 300 μg anti-GITR or control antibody and were immunized 30 minutes later with Ova323-339 in alum at the time of cecal ligation and puncture or sham surgery. Five days later, lymph node cells were harvested and 2.5 × 104 CD4+ cells were cultured with 2.5 × 105 irradiated APCs with Ova peptide for 72 hours. (A) Proliferation of CD4+ T cells was measured as the incorporation of 3H-thymidine during the last 18 hours of culture. Media was harvested at the time of 3H-thymidine addition to assess cytokine production. (B) IL-2, (C) IFN-γ, (D) IL-4, and (E) IL-10 concentrations were assessed by Luminex multiplex analysis. (F) Representative examples of flow plots demonstrating the expansion of DO11.10 T cells (KJI-26+CD4+) from lymph nodes of Balb/c mice that were injected intravenously with 5 × 106 DO11.10 CD4+ T cells 3 days before sham (left), CLP with control antibody (middle), or CLP with anti-GITR antibody (right) treatment at the time of immunization with Ova323-337 peptide in alum. (G) The calculated percentage of living (Sytox Blue−), nondebris DO11.10 T cells (KJI26+CD4+) expanded in the peripheral lymph nodes of the Balb/c mice. (H) The calculated total number of live (Sytox Blue−), nondebris DO11.10 T cells using cell counts obtained with a hemacytometer. P values indicate differences between groups after post hoc analysis using the Fisher LSD method.
To determine whether anti-GITR treatment improves antigen-specific CD4+ T-cell function, 2 experiments were performed. First, DO11.10 mice treated with anti-GITR or control antibody were immunized at the time of sepsis with 100 μg NP-Ova in alum. Five days later, lymph node cells were harvested and a proliferation assay was performed. We found that CD4+ T cells from anti-GITR–treated mice demonstrated an increase in proliferation comparable to the levels seen in sham mice (Figure 3A). Furthermore, anti-GITR treatment completely restored IL-2 production (Figure 3B), partially restored IFN-γ production (Figure 3C), and also augmented the TH2 cytokines IL-4 (Figure 3D), IL-10 (Figure 3E), and IL-5 (data not shown), although the latter 2 cytokines did not reach statistical significance.
Since we found that the total number of CD4+ T cells in the lymph nodes was higher in anti-GITR–treated septic DO11.10 mice (data not shown), we wished to see whether in vivo expansion of antigen-specific T cells was likewise improved by anti-GITR treatment. DO11.10 T cells were transferred into Balb/c mice 3 days prior to CLP or sham surgery and immunization with Ova(323-339) in alum. Seven days later, lymph nodes were harvested and the numbers of CD3+CD4+ T cells bearing the transgenic KJI-26 receptor were analyzed by flow cytometry. We found that anti-GITR treatment partially increased the percentage (Figure 3F,G) and almost completely restored the total number (Figure 3H) of KJI-26+CD4+ T cells to sham-treated levels. This improvement in antigen-specific expansion confirms that anti-GITR treatment can improve antigen-specific CD4+ T-cell function in vivo and indicates that the improvement of T-cell helper function was likely the reason for the improvement in humoral immunity following immunization in anti-GITR–treated septic mice.
Anti-GITR treatment decreases bacteremia and improves survival in sepsis
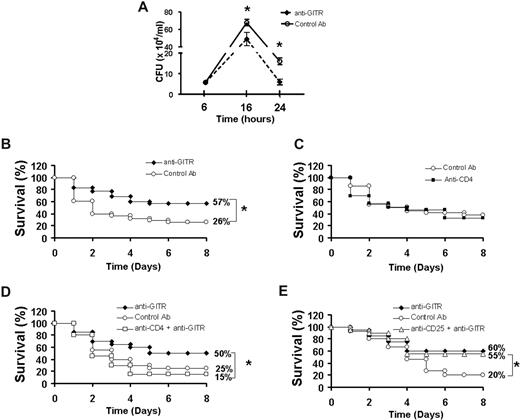
Since preventing T-cell apoptosis in mouse models has been shown to improve bacterial clearance, we examined whether anti-GITR improvement of T-cell function also decreases bacterial load.1 We found that CLP caused a profound bacteremia beginning as early as 6 hours and peaking approximately 14 to 18 hours after initiation (Figure 4A). Bacterial counts were still considerably elevated 24 hours after CLP. Mice that were given anti-GITR antibody demonstrated a 30% reduction in bacteremia at 16 hours after sepsis initiation and greater than 75% reduction in bacteremia at 24 hours after sepsis (Figure 4A; P < .05 by Student t test), indicating that anti-GITR treatment reduces both peak bacteremia and the speed of clearance of the bacteria.
Anti-GITR treatment decreases bacteremia and improves sepsis survival. (A) C57Bl/6 mice were given an intraperitoneal injection of 300 μg anti-GITR (n = 9) or control antibody (n = 9) 30 minutes before cecal ligation and puncture (CLP) surgery and mice were killed at 6, 16, or 24 hours afterward. Bacteremia was determined from blood obtained aseptically via cardiac puncture plated on sheep blood agar plates. Data are represented as mean ± standard error. (B) C57Bl/6 mice were given an intraperitoneal injection of 300 μg anti-GITR (n = 35) or control antibody (n = 38) 30 minutes before CLP surgery and survival was monitored for 8 days. Figure is the combination of 2 separate experiments with similar results. (C) C57Bl/6 mice were given injections of CD4 depleting (n = 15) or control antibody (n = 20) as described in “Proliferation assay.” Survival after CLP was monitored for 8 days. (D) C57Bl/6 mice depleted of CD4 cells (n = 20) or not (n = 20) were given anti-GITR antibody 30 minutes before CLP surgery. Another group of non-CD4–depleted mice receiving control antibody was used as a control group. (E) C57Bl/6 mice depleted of CD25+ cells (n = 20) or not (n = 20) were given anti-GITR antibody 30 minutes before CLP surgery. Another group of non-CD25–depleted mice receiving control antibody was used as a control group.*P < .05 by Student t test (A) or Fisher exact test (B-E). All error bars indicate SD.
Anti-GITR treatment decreases bacteremia and improves sepsis survival. (A) C57Bl/6 mice were given an intraperitoneal injection of 300 μg anti-GITR (n = 9) or control antibody (n = 9) 30 minutes before cecal ligation and puncture (CLP) surgery and mice were killed at 6, 16, or 24 hours afterward. Bacteremia was determined from blood obtained aseptically via cardiac puncture plated on sheep blood agar plates. Data are represented as mean ± standard error. (B) C57Bl/6 mice were given an intraperitoneal injection of 300 μg anti-GITR (n = 35) or control antibody (n = 38) 30 minutes before CLP surgery and survival was monitored for 8 days. Figure is the combination of 2 separate experiments with similar results. (C) C57Bl/6 mice were given injections of CD4 depleting (n = 15) or control antibody (n = 20) as described in “Proliferation assay.” Survival after CLP was monitored for 8 days. (D) C57Bl/6 mice depleted of CD4 cells (n = 20) or not (n = 20) were given anti-GITR antibody 30 minutes before CLP surgery. Another group of non-CD4–depleted mice receiving control antibody was used as a control group. (E) C57Bl/6 mice depleted of CD25+ cells (n = 20) or not (n = 20) were given anti-GITR antibody 30 minutes before CLP surgery. Another group of non-CD25–depleted mice receiving control antibody was used as a control group.*P < .05 by Student t test (A) or Fisher exact test (B-E). All error bars indicate SD.
To determine whether anti-GITR treatment results in improved outcome during sepsis, mice were again injected intraperitoneally with anti-GITR (n = 35) or a control antibody (n = 38) 30 minutes before the CLP procedure, and survival was monitored for 10 days. Agonistic GITR antibody improved survival to cecal ligation and puncture–induced sepsis by 31% over mice treated with an isotype control antibody (P = .002 by Fisher exact; Figure 4B), indicating that augmenting adaptive immune system function can improve outcome in sepsis.
Although predominantly T-cell subsets including CD4+, CD8+, and natural killer (NK) T cells are activated by GITR signaling, other cells such as peritoneal and lung macrophages can also express GITR.14 We first determined whether depletion of CD4+ T cells altered outcome to sepsis. CD4+ T cells were depleted by an intraperitoneal injection of 500 μg anti-CD4–depleting antibody (GK1.5) 3 days before sepsis initiation followed by another dose of 250 μg of the same antibody. Similar to our previous study, we found that depletion of greater than 95% of the total CD4+ cells does not affect mortality in the CLP model (Figure 4C and Scumpia et al8 ). To determine whether CD4+ T cells are responsible for the survival improvement by anti-GITR treatment, we treated mice with the CD4-depleting antibody as described above in this paragraph, except mice were given the anti-GITR antibody (300 μg) at the time of the second CD4-depleting dose (30 minutes to 1 hour prior to CLP). We found that mice depleted of CD4+ T cells lost the survival benefit of anti-GITR treatment (P = .04 by Fisher exact anti-GITR vs CD4-depleted anti-GITR; Figure 4D). This demonstrates that anti-GITR improvement in survival depends on the presence of CD4+ T cells.
Since GITR is expressed on both CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells, we next examined which subset of T cells is necessary for the survival benefit of anti-GITR treatment. Depletion of Tregs was achieved by treating mice with the same doses of CD25-depleting antibody PC61 as was used for the depletion of CD4+ T cells. This regimen resulted in greater than 90% depletion of CD4+CD25+ T cells.8 When mice were depleted of CD25+ Tregs, anti-GITR treatment was still able to improve survival to CLP (P = .04 by Fisher exact test anti-GITR, CD25-depleted vs control antibody; Figure 4E). These data indicate that CD4+ T cells but not CD4+CD25+ Tregs are necessary for the survival benefit offered by anti-GITR treatment.
Discussion
Despite decades of intense research, severe sepsis continues to be associated with an unacceptably high mortality rate, with over 200 000 people expected to die annually.16 The incidence is predicted to increase by 1.5% per year, owing to aging of the population and the wider use of immunosuppressive agents. An important goal is to identify novel therapeutic targets that will be critically dependent on an improved understanding of sepsis pathology.
Using the CLP model of polymicrobial sepsis, we found that sepsis-induced humoral immune dysfunction is associated with significant CD4+ T-cell dysfunction. Antigen-specific CD4+ T cells from immunized septic mice demonstrated impaired in vivo expansion when compared with immunized sham-treated mice, produced less IL-2 and IFN-γ, and proliferated poorly upon antigenic restimulation after immunization. However, CD4+ T cells from septic mice are able to proliferate to mitogenic stimulation with PMA/ionomycin treatment, indicating that the sepsis-induced T-cell defect likely lies with an impaired ability to stimulate the TCR. Importantly, T cells from both the spleen and lymph nodes demonstrated a considerable reduction in CD3ζ chain expression, highlighting a possible mechanism of TCR-mediated dysfunction in acute sepsis. CD3ζ is a master regulator of TCR signaling. Loss of CD3ζ expression has been documented in infectious, inflammatory, autoimmune, and malignant diseases, suggesting that it may serve to limit T-cell reactivity and effector cell responses at sites of tissue damage.17 Under conditions of chronic inflammation or stress, many factors have been attributed to the down-regulation of CD3ζ chain including TNF-α,18 oxidative stress,19 arginine depletion,20 and myeloid suppressor cells.21 Interestingly, acute exposure to agents that cause oxidative stress can also decrease CD3ζ chain expression in T cells,19 as can neutrophilic cells that are induced in the spleen by traumatic injury.22 This led us to hypothesize that the acute “cytokine storm” and oxidative burst that is a common early response to sepsis or the systemic inflammatory response may be detrimental to the development of T-cell responses and cause CD3ζ chain down-regulation, possibly as a self-regulating immunomodulatory mechanism or an unwanted side effect of sepsis-induced immune dysfunction.
Although T-cell dysfunction is known to occur in sepsis, much of the attention has been focused on the inability to produce TH1 cytokines and the propensity to produce TH2-type cytokines, but what is happening to T-cell populations in sepsis was largely overlooked. In a previous study, we found that Tregs are increased proportionally in sepsis and their ex vivo suppressive activity is enhanced, but their depletion does not alter sepsis mortality.8,23 This is in contrast to the findings of Heuer et al24 who found that adoptive transfer of ex vivo–activated Tregs can improve outcome in sepsis. The reason for the difference could be explained by the fact that the in vivo cytokine milieu produced during sepsis (including IL-1β and IL-6) may actually suppress the in vivo function of endogenous Tregs,25,26 which can be overcome by ex vivo stimulation of isolated T cells through CD3, CD28, and IL-2.
Interestingly, we also found that depletion of all CD4+ T cells does not affect survival in sepsis.8 An apparent paradox exists because the depletion of CD4+ T cells does not affect mortality in sepsis but prevention of T-cell apoptosis does improve survival in sepsis.1 In our previous study, and again in this study, we found that as early as 24 hours after sepsis initiation, T cells from septic mice are hyporesponsive to stimulation, similar to that seen in patients suffering from intra-abdominal sepsis.27 These T cells are characterized by the inability to proliferate to antigen-specific or nonspecific TCR-mediated stimulation and a failure to produce IL-2 and IFN-γ. These results may in part explain why the depletion of poorly functioning T cells does not affect outcome in sepsis, but increasing their function by preventing their apoptotic death or by costimulation through an agonistic GITR antibody may improve their function, leading to improvement in outcome.
GITR signaling has a dual effect on T cells whereby it can abrogate Treg-mediated suppression as well as costimulate T-effector cell activity.9,10 GITR is found at high levels on CD4+CD25+ regulatory T cells, but GITR expression is also found at lower levels on T-effector cells and increases further on T cells following activation. The beneficial effects of anti-GITR treatment on T-cell function also led to a CD4+ T-cell–dependent improvement in survival, as the improvement was abolished in mice that were previously depleted of CD4+ but not CD25+ T cells. An effective host response to invading pathogens entails a coordinated interplay between cells of the innate and the adaptive immune systems. CD4+ T cells produce key cytokines and chemokines that can activate and attract macrophages and neutrophils to sites of infection. Furthermore, T-cell interactions with B cells are important for the production of opsonizing or neutralizing antibodies to pathogens. A decrease in T-cell cytokine production, as found in this study, coupled with the apoptotic loss of T cells, will affect multiple facets of innate and adaptive immunity, both acutely and chronically, leading to uncontrolled infection or death. This study highlights the importance of maintaining T-cell function as a mechanism to sustain immune system function during a systemic bacterial infection and also challenges the dogma that T cells, and the adaptive immune system in general, require several days to begin functioning. In light of recent evidence that T cells express toll-like receptors on their cell surface and can respond to toll-like receptor agonists rapidly,28-30 the data in the present manuscript support the notion that T cells acting rapidly can impart protective immunity, especially if they are activated, as in this case, by an agonistic GITR antibody. Importantly, our findings that anti-GITR treatment led to improvements in T-cell proliferative and cytokine responses following stimulation ex vivo as well as a decrease in bacteremia in vivo strongly suggest that anti-GITR–treated mice mount a more effective response to the invading pathogen.
Our data also suggest that the interaction between the innate and adaptive immune system is both rapid and essential for survival in the early stages of sepsis. The implications of these results are 2-fold. First, it implies that T-helper cell dysfunction in sepsis is detrimental for host survival. Second, it implies that correction of this dysfunction with T-cell–specific therapies may lead to improved outcome in sepsis either by helping eliminate the primary infection or by preventing immunoparalysis that may lead to secondary infections.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant R37 GM-40586, awarded by the National Institute of General Medical Sciences (United States Public Health Service, T32; M.J.D., R.D.W.).
We thank Amer Abouhamze and Ricardo Ungaro for help with the multiplex cytokine assay.
Authorship
Contribution: P.O.S. designed and performed research, analyzed and interpreted data, and drafted the manuscript; M.J.D. performed research and helped draft the manuscript; K.M.K.-S. and J.S.W. performed research and analyzed data; J.L.W. performed research and helped draft the manuscript; C.X. provided novel research tools; R.D.W. and C.S.C. performed research; A.A. and M.A.A. provided novel research tools; W.H.R. provided research tools and interpreted data; M.J.C.-S. provided novel research tools and interpreted data; L.L.M. designed research, provided research tools, interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lyle L. Moldawer, Department of Surgery, University of Florida College of Medicine, 1600 SW Archer Road Room 6116, Gainesville, FL 32610-0286; e-mail:moldawer@surgery.ufl.edu.