To the editor:
Immunomodulatory drugs like thalidomide and lenalidomide are being increasingly used for the treatment of patients with multiple myeloma and myelodysplastic syndromes. In contrast to thalidomide, which undergoes nonenzymatic hydrolysis in the plasma, approximately two-thirds of lenalidomide is eliminated unchanged through the kidneys in healthy volunteers. The risk of adverse reactions is expected to be greater in lenalidomide-treated patients with impaired renal function.1,2 We would like to alert clinicians to the possibility of lenalidomide-induced hepatotoxicity when the drug is given in the setting of mild renal impairment after observing this rare side effect in a patient with multiple myeloma.
A 57-year-old African American male presented with weight loss and multiple lytic lesions. Laboratory evaluation revealed an elevated serum creatinine level (380.12 μM [4.3 mg/dL]), hypercalcemia (4.05 mM [16.2 mg/dL]), anemia (70 g/L [7 g/dL]), monoclonal gammopathy with depressed quantitative immunoglobulins (IgG, 7.68 g/L [768 mg/dL]; IgA, 0.54 g/L [54 mg/dL]; and IgM, 0.4 g/L [40 mg/dL]), and free kappa light chains on serum immunofixation. Bone marrow biopsy showed 90% kappa-restricted plasma cells consistent with stage IIIB multiple myeloma. He had no prior history of liver or kidney disease.
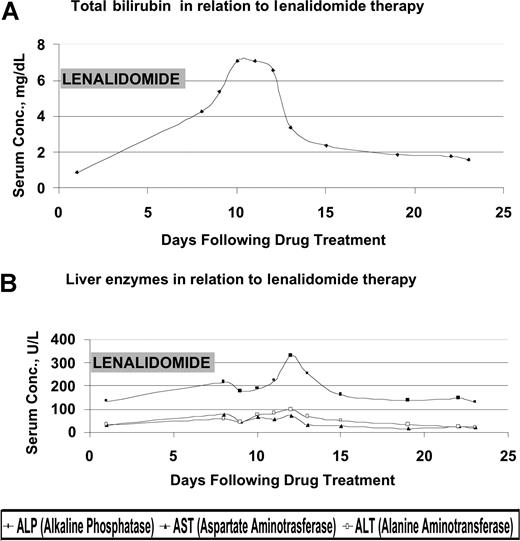
He began treatment with thalidomide (200 mg daily) and dexamethasone for 2 cycles but subsequently developed peripheral neuropathy and fatigue. His disease remained refractory to treatments including vincristine, adriamycin, and dexamethasone (VAD); bortezomib; and autologous stem cell transplantation. He was hospitalized for hypercalcemia, which improved from 0.45 mM to 0.275 mM (1.8 mg/dL to 1.1 mg/dL) after conservative management, and renal insufficiency. The patient was started on lenalidomide 25 mg orally daily and dexamethasone 40 mg orally on days 1 to 4, with warfarin 1 mg and aspirin 325 mg as venous thromboembolism prophylaxis. He was not receiving any hepatotoxic medications. One week later, he developed significant fatigue and hyperbilirubinemia (Figure 1). Lenalidomide was considered as a potential source of the liver function test abnormalities and discontinued. Further evaluation included an abdominal sonogram that showed mild hepatomegaly (16.5 cm) and 2 incidental hemangiomas (< 2 cm) but no bile duct dilatation or thrombi in hepatic veins. An abdominal magnetic resonance imaging (MRI) confirmed the hemangiomas but did not reveal any other specific pathology. He had complete normalization of his liver function profile 16 days following discontinuation of lenalidomide.
Effect of lenalidomide treatment on liver function tests. (A) Total bilirubin level in relation to lenalidomide therapy. (B) Liver enzymes in relation to lenalidomide therapy. Conc. indicates concentration.
Effect of lenalidomide treatment on liver function tests. (A) Total bilirubin level in relation to lenalidomide therapy. (B) Liver enzymes in relation to lenalidomide therapy. Conc. indicates concentration.
In a review of lenalidomide toxicities by Hussein,3 grade 2 liver enzyme elevations were described in 1 patient in a single-agent lenalidomide study. There was a similar association with fatigue, and a liver biopsy showed no specific pathology. Elevations in liver enzymes recurred after the patient was rechallenged with a lower dose.
It is possible that our patient's reduced renal function played a role in the development of hepatotoxicity. The development of hepatotoxicity in this patient and its subsequent improvement coincided with the initiation and discontinuation of lenalidomide. This provides a temporal relationship and the basis for a strong association of hepatotoxicity induced by lenalidomide. As use of this drug expands, clinicians must recognize hepatotoxicity as a rare but potential complication of lenalidomide therapy. Since the drug is excreted mainly via the kidney as unchanged drug, higher caution would be warranted in patients with preexisting liver or kidney disease.
Authorship
Correspondence: Subuhee Hussain, Department of Oncology, Montefiore Medical Center, 111 E 210th St, Bronx, NY 10467; e-mail:shussain@montefiore.org.