Abstract
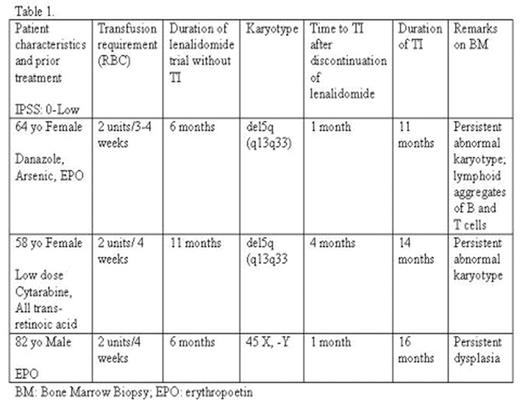
Lenalidomide was approved by the US Food and Drug Administration in December of 2005 for the treatment (tx) of transfusion-dependent anemia (TDA) in patients (pts) with MDS and chromosome 5q deletions (del5q). In the trial that secured the approval, 67% of 148 pts achieved red blood cell (RBC) transfusion independence (TI) with a median time to TI of 44 weeks. 90% of pts who achieved TI did so by completion of 3 months of therapy. In a companion study (MDS-002) pts lacking del5q the frequency of TI was only 26%. We report three cases of IPSS Low risk myelodysplasia MDS with a delayed response to lenalidomide. The patient characteristics are outlined in Table 1.
Following a 6 to 11 month trial of lenalidomide for TDA in IPSS Low risk MDS delayed TI can occur 1 to 4 months after discontinuation of lenalidomide. Delayed TI was not associated with a cytogenetic response. The duration of response can last from 11 months to 16 months. Lenalidomide, a thalidomide analogue, is an immunomodulatory agent with anti-angiogenic and anti-neoplastic properties. Effects of lenalidomide such as increased production of interleukin-2 and interferon-γ have been linked to the appearance of lymphoid aggregates of a mixture of B and T cells in the BM specimens of responders. TI has previously been correlated with suppression of the del5q clone. In our patients, TI was achieved after discontinuation of lenalidomide following a reasonable ineffective trial and no apparent suppression of the del5 clone. A similar phenomenon was described by Raza, et al. in a single MDS patient treated with thalidomide. Interestingly, one patient had lymphoid aggregates of B and T cells throughout treatment, raising the possibility that the immune stimulatory effects of lenalidomide may be a variable against the del5q clone. The transient response may be due to immunomodulatory effects but persistence of the del5q clone in two of our patients and and dysplasia in the other, is responsible for the relapse after the immune response has waned. Re-treatment with lenalidomide in our patient did not prolong or re-induce another response. References Giagounidis AAN, Germing U, Aul C: Biologic and prognostic significance of chromosome 5q deletions in myeloid malignancies. Clin Cancer Res 2006, 12:5–10. List A, Kurtin S, Roe DJ, et al.: Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005, 352:549–557. List A: Recent advances in the treatment of MDS. Clin Advances in Hematology. Oncology. 2007, Supp 10, Vol 5, Issue 7: 4–5 Raza A, Meyer P, Dutt D, et al. Thalidomide produces transfusion independence in long-standing refractory anemias of patients with myelodysplastic syndromes. Blood. 2001 Aug 15, 98(4):958–65.
Disclosure:Research Funding: Investgator initiated clinical trial. Membership Information: Advisory Board and Speaker’s Bureau for Celgene.