Abstract
Introduction: Natural killer (NK) cell activity is regulated by a dynamic balance between inhibitory and activating receptors that recognize ligands on target cells. Human leukocyte antigen (HLA)-class I, particularly HLA-C and -Bw4 molecules, are key ligands transmitting inhibitory signals to NK cells. NK cells avidly lyse tumor cells that do not display such inhibitory KIR-ligands. The proteasome is responsible for the generation of peptides that bind to and stabilize class I molecules at the cell surface. We hypothesized that bortezomib, a partial proteasome inhibitor that is clinically approved for the treatment of refractory/relapsed myeloma (MM), could reduce HLA expression on MM cells and thus enhance NK cell-mediated cytotoxicity.
Methods: HLA-class I or HLA-C expression was assessed using flow cytometry, after gating on AnnexinV/PI double negative cells, and/or confocal microscopy. Expression of other proteins was measured by flow cytometry using specific mAb. NK cell-mediated lysis of myeloma cells was measured by 51Cr-release.
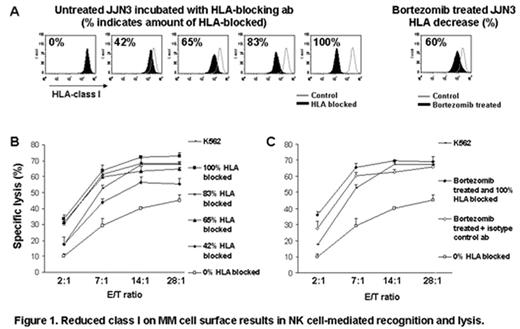
Results: Bortezomib at clinically attainable concentrations down-regulated HLA-class I expression on MM cells in a time- and dose-dependent fashion. Reduction of class I post-10 nM bortezomib treatment was observed in all myeloma cell lines tested (n=10), by a median of 49% (range: 19–66%). A similar decrease of HLA-class I was obtained in 10–50 nM bortezomib treated primary MM cells (n=6). Bortezomib significantly enhanced the sensitivity of MM cells to allogeneic and autologous NK cell-mediated lysis. Further, the level of reduction in HLA-class I expression correlated well with increased susceptibility to lysis by NK cells. The level of down-regulation of HLA-class I induced by bortezomib was reproduced by incubating MM cells with HLA-blocking antibody and resulted in equipotent enhancement of NK cell-mediated lysis (Figure 1). The extent of HLA-class I down-regulation by bortezomib was therefore biologically relevant. Down-regulation of HLA-class I was also observed in vivo on purified MM cells 48 hours after a single dose of bortezomib, by a median of 47% (range: 16–63%, n=6, P= .002). HLA-C expression (the principal NK cell inhibitory ligand) was rescued by exogenous provision of HLA-C binding peptides providing a mechanistic explanation for the effect of bortezomib on HLA-class I expression. Finally, we did not observe bortezomib-mediated enhancement of NK cell-mediated lysis of myeloma through receptors other than the KIR receptor family, including tumor necrosis factor related apoptosis-inducing ligand, NKG2D and natural cytotoxicity receptors. HLA-class I down-regulation was not observed in renal cell and breast carcinoma cell lines, which is in keeping with the remarkable activity of bortezomib in myeloma. Our findings have clear therapeutic implications for MM and other NK cell-sensitive malignancies in the context of both allogeneic and autologous adoptively transferred NK cells.
Reduced class I on MM cell surface results in NK cell-mediated recognition and lysis
Reduced class I on MM cell surface results in NK cell-mediated recognition and lysis
Author notes
Disclosure:Research Funding: Bart Barlogie recieves research funding from Millenium. Bortezomib for these studies was provided by Millenium.