Abstract
Introduction: Inorganic polyphosphate (polyP) is a negatively charged polymer of phosphate units linked by high energy phosphoanhydride bonds. Dense granules of human platelets contain abundant polyP which is released in response to thrombin stimulation. We recently reported that polyphosphate is a potent hemostatic regulator, accelerating blood clotting by activating the contact pathway, promoting factor V activation, and abrogating tissue factor pathway inhibitor function. We now report that polyP shortens the clot time of hemophilic plasmas and plasmas containing a variety of anticoagulant drugs.
Methods: Citrated plasmas were obtained from normal donors, patients receiving coumadin, and individuals with severe hemophilia A or B. In some experiments, clinically relevant concentrations of unfractionated heparin, enoxaparin, or argatroban were added to normal plasma. In other experiments, therapeutic concentrations of FIX, FVIII, or FVIIa were added to hemophilic plasma. Clotting was initiated by dilute thromboplastin.
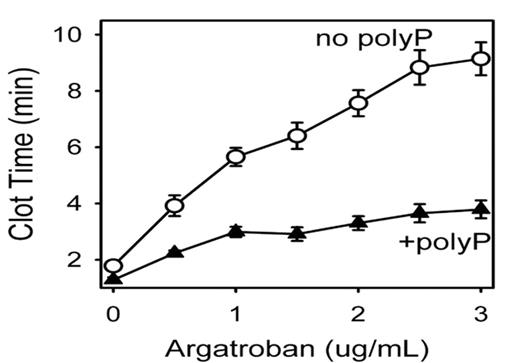
Results: Adding 33 uM polyP to normal plasma shortened clot times at all doses of heparin (by 37–55%), enoxaparin (by 35–57%), and argatroban (by 28–59%; see Figure). PolyP shortened clot times of plasmas from coumadin patients (by 29–39%) with INR values ranging from 1.7 to 4.8. PolyP also shortened the clot times of plasma from patients with hemophilia A (by 54–75%) and B (by 51–79%). PolyP alone shortened clot times of hemophilia A or B plasma equivalent to adding 6 to 15 nM recombinant FVIIa, and the polyP effect was additive to that of FVIIa, FVIII or FIX.
Conclusions: PolyP shortened the in vitro clot time for all plasmas tested and was additive with replacement of missing clotting factors or recombinant FVIIa for hemophilic plasmas. These results suggest that treatment with polyP may be useful for reversal of anticoagulation in patients treated for thrombophilia, or for treatment of bleeding episodes in patients with hemophilia. Furthermore, polyP could potentially reduce the dose of replacement clotting factors or recombinant FVIIa needed to treat bleeding episodes in hemophilia.
Author notes
Disclosure: No relevant conflicts of interest to declare.