Abstract
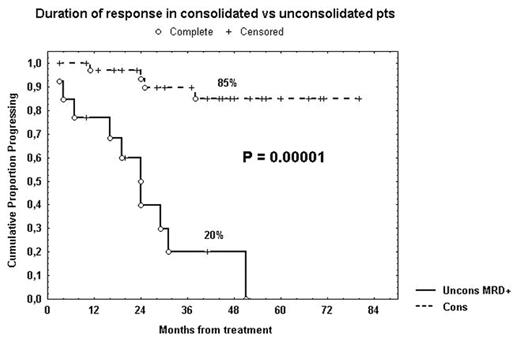
Monoclonal antibodies in combination with chemotherapy allowed us to obtain more responses and longer response duration in B-cell chronic lymphocytic leukemia (B-CLL), reducing disease burden to levels detectable only by flow cytometry. Moreover, it has been reported that low-dose rituximab decreases CD20 antigen loss via shaving and promotes enhanced targeting in CLL (Williams, 2006). We performed a phase II study that added rituximab to fludarabine (Flu) as therapy for symptomatic, untreated CLL. Remission status was assessed by a multiparametric flow cytometric method based on the detection of CD19+CD5+CD79b– residual B-CLL lymphocytes. VH mutational status, CD38, ZAP-70 and cytogenetics were obtained in all pts before treatment. We defined as “high risk” pts having at least two of the following markers: unmutated IgVH, CD38>30%, ZAP-70>20%, intermediate/unfavorable cytogenetics (trisomy 12 or del11q or del17p). Eighty-two CLL pts, median age 61 years, received six monthly courses of Flu (25 mg/m2 for 5 days) and four weekly doses of rituximab (375 mg/m2) starting after completion of Flu therapy. According to modified Rai stages, 8 pts had a low stage, 70 an intermediate stage and 4 a high stage. Based on NCI criteria, 66/82 (80%) pts achieved a complete remission (CR), 12/82 (15%) a partial remission (PR) and 4/82 (5%) no response or progression. Hematologic toxicity included mainly neutropenia (grade 3 and/or 4 in 42 pts) and thrombocytopenia (grade 3 and/or 4 in 4 pts). Thirty-five pts in clinical CR or PR, either with CD5+CD19+CD79b– bone marrow (BM) cells >1% (MRD+, n=20 pts) or MRD negative but presenting CD5+CD19+ peripheral blood lymphocytes (PBL) >1000/microl (n=15 pts) within 1 year after completion of the induction treatment, underwent consolidation/maintenance therapy with four monthly cycles of rituximab at 375 mg/m2 followed by twelve monthly doses of rituximab at 150 mg/m2. The median follow-up duration was 46 months. Noteworthy, all B-CLL pts experienced a long progression-free survival (PFS) from the end of induction treatment (68% at 5 years). Nevertheless, CLL pts that underwent consolidation and maintenance therapy (n=35) showed a significant longer response duration (85% at 5 years, Figure). On the other hand, BM and PBL persistently MRD negative (>1 year) pts (n=29) showed a response duration similar to that of the consolidated pts (87% at 5 years). A significant shorter PFS was observed within CD38+ pts (39% vs 78% at 5 years, P=0.002), unmutated pts (45% vs 94% at 2.5 years, P=0.001) and ZAP-70+ pts (36% vs 88% at 6 years; P=0.00002). Notably, within the “high risk” subset (n=30), considering only MRD+ pts in CR or PR (n=20), MRD+ consolidated pts (n=11) showed a significant longer response duration (64% vs 13% at 2 years, P=0.006) in comparison with MRD+ unconsolidated pts (n=9). In conclusion, consolidation/maintenance therapy with rituximab prolongs significantly the response duration in B-CLL, improving also the outcome of the “high risk” subset.
Author notes
Disclosure: No relevant conflicts of interest to declare.