Abstract
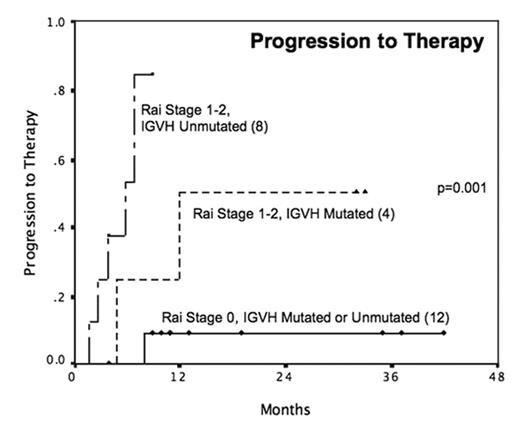
The adverse prognostic significance of chromosome17p deletion (del17p) was established in CLL patients (pts) with pretreated disease, and its significance in patients with previously untreated CLL (“de-novo del17p”) is not well defined. We identified 48 consecutive pts with de-novo del17p within a cohort of 708 previously untreated pts tested by FISH for common CLL aberrations and followed prospectively at the MD Anderson Cancer Center. Characteristics of de-novo 17p pts: median age 61 yrs; Rai 0 31%, I–II 44%, III–IV 25%; B2M≥2N 42%; unmutated IGVH 32/45. The proportion of del17p cells were ≥20% in 35 (73%) pts. Twenty-five (52%) pts had stable disease not requiring immediate therapy, and were observed prospectively; for these pts, the actuarial risk of requiring therapy was 41% at a median follow-up of 19 months. Pts in Rai stage 0 had a low risk of progression to therapy (9%) regardless of IGVH status, whereas the progression risk for Rai I–II pts depended on their IGVH status (figure). Thirty-two pts had commenced therapy, of which 25 were assessable for treatment response (table). Overall (OR) and complete clinical response (CCR) rates were 67% and 33% for rituximab-based therapy, and 84% and 50% for fludarabine & rituximab combinations. Bone marrow examination in 8 CCR pts confirmed that 7 were in flow cytometry negative CR, with normalization of cytogenetics and FISH in 6 of 6 tested. Time to progression for CCR pts was 79% at 18 months, with only 3 relapses to date: notably, 2 relapses were with Richter transformation (one of which was confirmed to be del17p negative), and the only indolent relapse was with a non-del17p clone. Survival for all pts (n=48) from FISH date was 85% at 18 months, and survival from start of therapy (n=32) was 86% at 18 months. Five deaths have occurred to date: two pts died of unrelated causes prior to requirement for CLL therapy, two pts in remission died of complications of therapy, and one pt died of obstructive jaundice related to an undiagnosed pancreatic mass. De-novo del17p does not necessarily imply a dismal prognosis in CLL: the disease course may be indolent especially in Rai 0 disease, and therapy with standard regimens can achieve durable clinical and cytogenetic remissions. All pts who commenced therapy in this study received rituximab, and our favorable survival compared to that reported by the German and United Kingdom trials of F vs FC (median survival 18 months or less) suggest that rituximab may be an important agent in the therapy of del17p CLL.
Figure
| . | Evaluable . | Response . | Comp Clin Resp . | Flow–CR . | Remission (months) . |
|---|---|---|---|---|---|
| Rit & GMCSF | 4 | 2 | 1 | 1 | 9, 18+ |
| Rituximab | 1 | 1 | 1 | n/a | 42+ |
| Rit & Pred | 1 | 1 | 0 | 0 | 3+ |
| RIT-BASED | 6 | 4 (67%) | 2 (33%) | 1/5 (20%) | 53% @ 18mth |
| Evaluable | Response | Comp Clin Resp | Flow- CR | Remission (months) | |
| FCR ±GMCSF | 11 | 10 | 5 | 3/9 | 2+,6+,8+,9+,11+,12+,15,22+,22,41+ |
| FCMR | 2 | 1 | 1 | 0 | 3+, 18+ |
| FR | 1 | 0 | 0 | 0 | n/a |
| PCR | 1 | 1 | 1 | 1 | 19+ |
| CFAR | 4 | 4 | 2 | 2 | 11,11,12,12+ |
| 19 | 16 (84%) | 9 (50%) | 6/17 (35%) | 62% @ 18mth |
| . | Evaluable . | Response . | Comp Clin Resp . | Flow–CR . | Remission (months) . |
|---|---|---|---|---|---|
| Rit & GMCSF | 4 | 2 | 1 | 1 | 9, 18+ |
| Rituximab | 1 | 1 | 1 | n/a | 42+ |
| Rit & Pred | 1 | 1 | 0 | 0 | 3+ |
| RIT-BASED | 6 | 4 (67%) | 2 (33%) | 1/5 (20%) | 53% @ 18mth |
| Evaluable | Response | Comp Clin Resp | Flow- CR | Remission (months) | |
| FCR ±GMCSF | 11 | 10 | 5 | 3/9 | 2+,6+,8+,9+,11+,12+,15,22+,22,41+ |
| FCMR | 2 | 1 | 1 | 0 | 3+, 18+ |
| FR | 1 | 0 | 0 | 0 | n/a |
| PCR | 1 | 1 | 1 | 1 | 19+ |
| CFAR | 4 | 4 | 2 | 2 | 11,11,12,12+ |
| 19 | 16 (84%) | 9 (50%) | 6/17 (35%) | 62% @ 18mth |
Author notes
Disclosure: No relevant conflicts of interest to declare.