Abstract
Background: Vorinostat is a histone deacetylase inhibitor that has been approved by the US FDA for the treatment of cutaneous manifestations in patients with cutaneous T-cell lymphoma (CTCL) who have progressive, persistent or recurrent disease on or following 2 prior therapies.
Patients and Methods: A Phase IIb, open-label trial of oral vorinostat 400 mg daily until progressive disease (PD) or intolerable toxicity was conducted in patients with advanced CTCL. Patients must have received at least 2 prior therapies and recovered from all prior treatment-related toxicities. The primary end point was the objective response rate in patients with stage IIB or higher CTCL as measured by the severity weighted assessment tool. Time to response (TTR), duration of response (DOR), pruritus relief, safety, and tolerability were also evaluated. Patients who had received vorinostat therapy for at least 2 years as of August 1, 2007 were further evaluated in this post-hoc subset analysis.
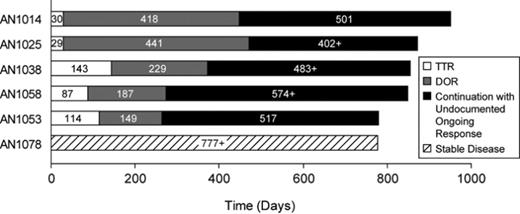
Results: Six of 74 patients originally treated in the study have received vorinostat therapy for at least 2 years as of 8/1/07, including 5 responders (1 complete responder, 4 partial responders) and 1 with prolonged stable disease (Figure 1). The median age was 65 years (range, 57–74), the median number of prior systemic therapies was 2.5 (range, 1–5), and the median time from CTCL diagnosis to enrollment was 1.8 years (range, 0–5.9). The most common drug-related adverse experiences were diarrhea (100%), fatigue (67%), nausea (50%), and alopecia (50%). Grade ≥ 3 drug-related adverse experiences were anorexia (n = 1), pulmonary embolism (PE, n = 1), and thrombocytopenia (n = 1). The PE (Day 144) was a serious adverse experience (SAE) that resolved within 7 days, and this patient is continuing on therapy as of Day 848. The only other SAE was a rash (Day 925). Two patients discontinued due to PD and 4 are continuing on therapy as of August 1, 2007.
Conclusions: Vorinostat has demonstrated prolonged safety and clinical benefit in patients with advanced CTCL.
Time on study of patients on vorinostat therapy for ≥ 2 years (available data as of August 1, 2007).
Time on study of patients on vorinostat therapy for ≥ 2 years (available data as of August 1, 2007).
Author notes
Disclosure:Employment: Justin L. Ricker, Syed Rizvi, Cong Chen, Kathleen Boileau, and Paulette Cooley are employees of Merck Research Laboratories. Consultancy: Madeleine Duvic, Elise A. Olsen, and Theresa R. Pacheco-Merck & Co., Inc. Ownership Interests:; Sareeta Parker, Justin L Ricker, Syed Rizvi, Cong Chen, Kathleen Boileau, Paulette Cooley-Merck & Co., Inc. Research Funding: Madeleine Duvic, Elise A. Olsen, Sareeta Parker-Merck Research Laboratories. Honoraria Information: Madeleine Duvic - Merck & Co., Inc. Membership Information: Larisa J. Geskin - Merck & Co., Inc.