Abstract
Background: Results from the International Randomized Study of Interferon and STI571 (IRIS) trial showed that achievement of a CCyR is prognostically relevant for long-term survival in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP). With the advent of next generation tyrosine kinase inhibitors (TKIs), there is a need to understand factors that may influence long term outcomes. Here we analyze whether time to achievement of a CCyR affects long term outcomes.
Methods: The relationship between time to CCyR and long-term outcomes was examined for 551 imatinib-treated patients with newly diagnosed CML-CP at the 6-year follow up of the IRIS trial. As evaluations included non-responders, landmark analyses were conducted in which only pts who were treated for ≥1 year were included (n=509). Patients were stratified according to time to achieving a CCyR as follows: ≤6 months (n=265), >6≤12 months (n=99), >12≤18 months (n=34), >18 months (n=49), or no CCyR during imatinib treatment (n=62). In each category patients were assessed for duration of cytogenetic response, event free survival (EFS; any event while on study), freedom from progression to accelerated phase (AP) or blast crisis (BC), and overall survival (OS).
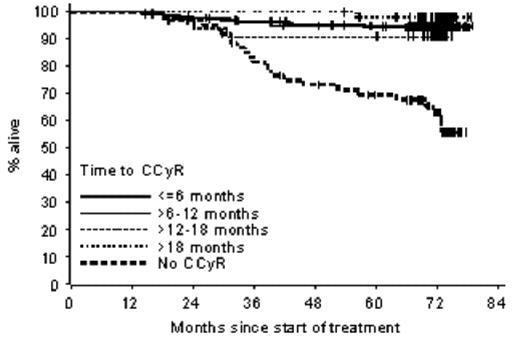
Results: In the 447 patients who were treated for at least 1 year and achieved a CCyR (<1% Ph+) during therapy, the durability of major cytogenetic response (1%–35% Ph+) did not differ significantly regardless of when CCyR was achieved (P=0.76). For the overall population, estimated 6-year rates were 88% for OS, 83% for EFS, and 93% for freedom from progression to AP/BC. No statistically significant difference was observed between the responders when categorized according to time to response. However, patients who did not achieve a CCyR had significantly worse outcomes than those who achieved CCyR (P<0.001). Estimated 6-year OS rates were 94%, 95%, 91% and 98% for patients who first achieved a CCyR within 6, 12, 18 months and after 18 months, respectively (P=0.55 for overall comparison of the response categories), compared with 63% for pts without CCyR during imatinib therapy. Estimated EFS rates at 6 years were 93%, 90%, 87% and 89% (P=0.58) and 33% for patients who do not achieve a CCyR, respectively. At 6 years the estimated rates of freedom from progression to AP/BC were 97%, 97%, 97% and 98% (P=0.98), respectively, but only 63% for pts who do not achieve a CCyR. Overall Survival by Time to CCyR.
Conclusion: Long-term outcomes on imatinib for patients in CML-CP are independent of time to achieve CCyR. Therefore, achievement of a “late” CCyR does not increase the potential for progression or portend a worse overall survival for patients treated with imatinib.
Author notes
Disclosure:Employment: Gathmann--employee of Novartis. Consultancy: Guilhot - study committee for Novartis; Larson - Novartis. Ownership Interests: Gathmann - Novartis. Honoraria Information: Guilhot - scientific board speaker for Novartis; Larson - Novartis. Paid Export Testimony Information: Larson - Novartis.