Abstract
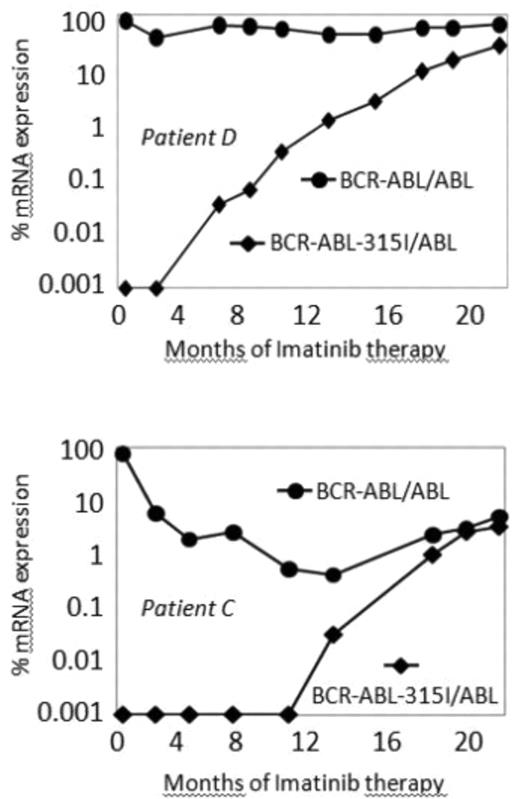
Imatinib mesylate (IM) is currently the first line treatment for patients with Chronic Myeloid Leukemia (CML) allowing induction of major molecular responses in the majority of patients. Nevertheless, resistance develops in a small proportion of patients principally due to missense mutations in the ABL-kinase domain of the BCR-ABL fusion gene. New drugs, such as Dasatinib or Nilotinib, which have recently been designed to overcome IM-resistant mutants, remain inefficient against the mutation at the gatekeeper position (T315I). Thus, this mutation is likely to become the most critical hurdle in patients who fail to benefit from ABL competitive inhibitors. Consequently, when a T315I mutation is detected, a quantitative monitoring of 315 mutants seems to be crucial to evaluate the response to new therapies. For that purpose, we developed a sensitive quantitative-RT-PCR method using allele specific primers to quantify mRNA harboring the T315I mutation. Each cDNA from blood samples were tested in duplicates for ABL, BCR-ABL and BCR-ABL-315I mRNA expression. Serial dilutions of a p210BCR-ABL-315I plasmid were used to construct standard curves allowing the determination of numbers of ABL, BCR-ABL and BCR-ABL-315I mRNA copies. Results were expressed as the BCR-ABL/ABL ratio and BCR-ABL-315I/ABL. To ensure the quality of the results, several controls were analyzed together with patient samples: distilled water, cDNA from healthy blood donors, cDNA from CML patients at diagnosis and cDNA from IM-resistant CML patients exhibiting a T315I mutation. Using this protocol, we retrospectively studied cDNA samples from 5 patients with an acquired resistance to IM therapy due to the presence of the T315I mutation. The periods of follow-up went from 6 months to 2 years. The BCR-ABL/ABL and BCR-ABL-315I/ABL ratios were determined for all cDNA samples and the kinetics for the two transcripts were carried out for each patient. The Q-RT-PCR method, with the standard curves and the different controls, appears to be efficient enough to allow a reliable investigation. Figure shows the results obtained for patient D and C. In patient D, showing a total resistance to IM, a slow and constant increase in the expression of the BCR-ABL-315I mRNA was observed. Conversely, in patient C, BCR-ABL-315I mRNA increased more rapidly after an initial IM-responsive phase. The differential kinetics observed between the two patients suggest that target stem cells for T315I mutation could have some hierarchical heterogeneity with regard to their emergence. Similar results were obtained for the 3 other patients. In conclusion, the quantitative RT-PCR assay that we developed to estimate the relative amount of BCR-ABL mRNA exhibiting the T315I mutation could be of major interest not only for the evaluation of the mechanism of resistance but also for the follow-up of T315I-bearing CML stem cells in patients undergoing targeted therapy using new non-ATP competitive BCR-ABL inhibitors.
Author notes
Disclosure: No relevant conflicts of interest to declare.