Abstract
Background: Primary systemic amyloidosis (AL) is an incurable plasma cell disorder with a poor survival, especially in patients (pts) with multiple organ involvement. For pts with limited organ involvement, high dose chemotherapy with autologous stem cell (SCT) support is an effective approach. However, the toxicity of SCT limits its widespread use. Melphalan and dexamethasone has been previously shown to be effective in pts ineligible for SCT.
Methods: We reviewed our experience with melphalan and dexamethasone for treatment of AL. Eighty one pts were treated between 2001 and 2003; median age at start of therapy was 63 years (range, 38–80) and 56 pts (69%) were males. Among these, 46 pts (57%) had 2 or more major organs (cardiac, renal, hepatic) involved. Intravenous melphalan (IV) was used in 45 pts (56%), 32 (40%) received oral melphalan (PO) and the rest received cycles of both. The most common doses were 16 mg/m2 for IV administration (every 4–6 weeks) and 0.22 mg/kg for PO administration (every 4 weeks). Dexamethasone was usually given at a dose of 40 mg for the first 4 days of each cycle.
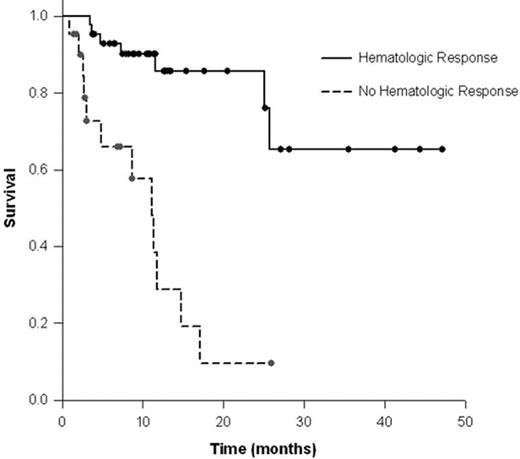
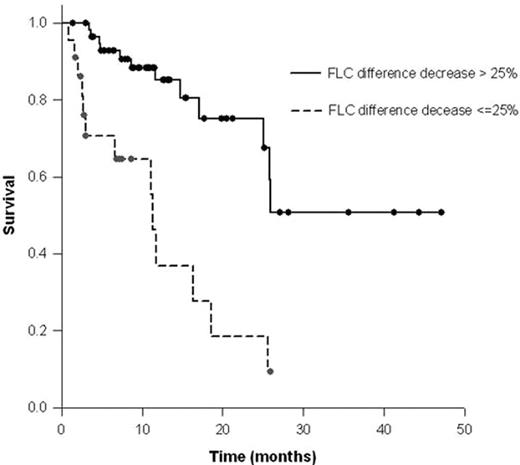
Results: Patients received a median of 5 cycles (range; 1–17). At the time of analysis, 53 (65%) pts were alive with a median follow up of 10.8 months (range, 1.4–47.1).The estimated median overall survival (OS) was 25.6 months for the entire group, with the number or type of organs involved not impacting survival. Hematological response (HR) was defined by a 50% reduction in either serum M-spike, urine M-spike, or the absolute difference between the involved and uninvolved immunoglobulin free light chains. A HR was observed in 43 pts (67%) of the 64 pts measurable for HR by conventional criteria. Achievement of HR was associated with a superior OS with a median post-therapy OS that was not reached, while non-responders had a median OS of 11.1 months (p<0.0001) (Figure 1). Among these pts, an organ response as defined by conventional criteria, was seen in 14 pts (22%), which was significantly correlated with obtaining a hematological response (33% vs. 5%, p=0.01). A 25% reduction in the difference between the involved and uninvolved FLC strongly predicted prolonged OS, with a median post-therapy OS that was not reached for responders vs. 11.3 months for non-responders (p<0.0001) (Figure 2). A decrease in post-therapy brain natriuretic peptide (BNP) also predicted for a survival improvement, with the median OS not reached for responders vs. a median OS of 17 months for non-responders (p<0.016).
Conclusion: Treatment with melphalan and dexamethasone is effective and a significant survival advantage is achieved in patients with a hematological response. FLC and BNP response can help identify patients that are likely to benefit from this therapy.
Author notes
Disclosure: No relevant conflicts of interest to declare.