Abstract
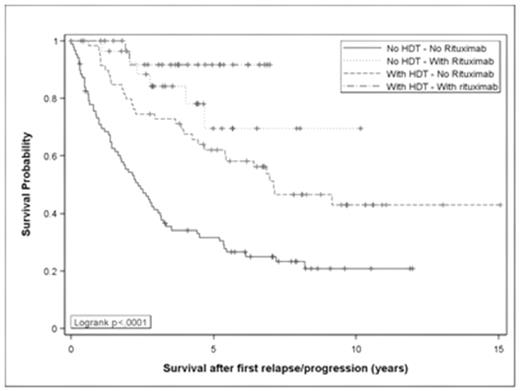
Follicular lymphoma (FL) is considered as an incurable disease but treatments have changed with the introduction of high-dose therapy with autologous stem cell transplant (HDT) then of rituximab (RTX). The effect of these 2 strategies on the outcome of FL patients is well described in first line but not for relapsing patients. We analyzed 2 cohorts of patients treated in 2 successive randomized studies with the same treatment, CHVP plus interferon, to evaluate the role of RTX and HDT in first disease progression or relapse. Of the 364 patients <61 years old included in these 2 studies, 255 progressed or relapsed and constitute the analyzed population. Among them, 93 had been treated with HDT, 63 with RTX alone or combined with chemotherapy, and 30 with RTX followed by HDT. Characteristics of the patients were similar in all subgroups. If event-free survival (EFS) was similar in patients treated within GELF-86 or GELF-94 studies(10-year EFS of 20% and 23%), overall survival (OS) was longer in GELF-94 study (10-year OS of 66% compared to 48%, p=0.014) witnessing a change in the management of relapsing patients. HDT was associated with a statistically significant benefit in terms of event-free survival from relapse (EFSR) and survival after relapse (SAR), p=0.004 and p=0.01, respectively (Table). Rituximab during salvage therapy was associated with a larger benefit than HDT for these 2 endpoints: p<0.0001 and p<0.0001, respectively. When both treatments were combined, 5-year SAR is over 90% (Table). In follicular lymphoma patients treated with chemotherapy in first line, the combination of salvage regimen containing rituximab followed by HDT seems to be superior to rituximab-containing chemotherapy alone. These retrospective results must be confirmed within a prospective study.
| . | Salvage without rituximab without HDT (A) . | Salvage without rituximab with HDT (B) . | Salvage with rituximab without HDT (C) . | Salvage with rituximab . |
|---|---|---|---|---|
| * p values between A and C =0.0002 and <0.0001 for EFSR and SAR, respectively; p values between B and D <0.05 and =0.01 for EFSR and SAR, respectively | ||||
| Patients | 87 | 59 | 29 | 30 |
| 5-year EFSR | 24% (15–34) | 44% (31–56) | 42% (21–62) | 67% (42–86) |
| 5-year SAR | 32% (22–42) | 62% (45–73) | 70% (42–86) | 92% (71–98) |
| . | Salvage without rituximab without HDT (A) . | Salvage without rituximab with HDT (B) . | Salvage with rituximab without HDT (C) . | Salvage with rituximab . |
|---|---|---|---|---|
| * p values between A and C =0.0002 and <0.0001 for EFSR and SAR, respectively; p values between B and D <0.05 and =0.01 for EFSR and SAR, respectively | ||||
| Patients | 87 | 59 | 29 | 30 |
| 5-year EFSR | 24% (15–34) | 44% (31–56) | 42% (21–62) | 67% (42–86) |
| 5-year SAR | 32% (22–42) | 62% (45–73) | 70% (42–86) | 92% (71–98) |
Figure
Author notes
Disclosure:Research Funding: Research funding from Genentech and Roche. Honoraria Information: Genentech and Roche.