Abstract
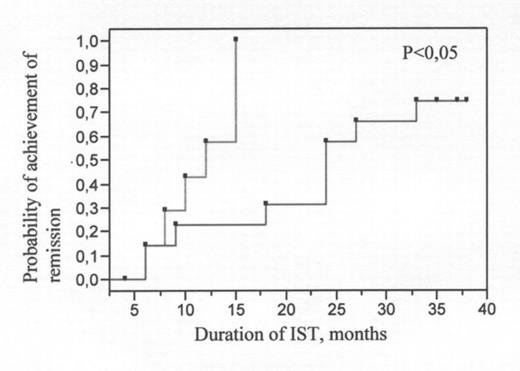
Dendritic cells (DCs) play principal role in the induction of antigen-specific T-cell immune response and in the development of antigen-specific self-tolerance. Moreover, they promote either Th1 or Th2 polarization of naive lymphocytes by production of immunoregulatory cytokines (IL-12 and IL-10). AA is characterized by increased Th1/Th2 ratio and by Th1 mediated antigen-specific suppression of hematopoiesis. DCs might be responsible for the activation of T-clone in AA patients. However, functional characteristics such as IL-12 production by DC in AA patients have not been studies yet. Therefore, IL-12 secretion capacity of DCs generated from peripheral mononuclear cells (PMNC) of 30 AA patients before and during the immunosuppressive therapy (IST) was compared to that of DCs of 7 donors. DC were derived from PMNC in the presence of GM-CSF and IL-4 for 5–7 days and further stimulated by exposition to 3T3-CD40L fibroblasts for 48 hours. Supernatants of DC cultures were subjected to ELISA for evaluation of IL-12 production. 70,8% of AA patients exhibited significantly (p=0,02) increased baseline level of IL-12 production by DCs compared to that of donors. Programmed IST resulted in the diminution of IL-12 production level in 67% of AA patients. Moreover, significant decrease in IL-12 production level correlated (Rs=0,79) with the achievement of independence from transfusions. Patients with high baseline level of IL-12 production by DCs required continuous (median - 24 months, p<0,05, see pict. 1) IST including repeated courses of antithymocyte globulin and cyclosporin-A. These data suggest that increase in IL-12 production by DCs of AA patients might contribute to cytotoxic T-clone expansion and consequently, to AA development. Patients that showed high initial levels of IL-12 secretion by DCs comprised the worst prognostic group.
Author notes
Disclosure: No relevant conflicts of interest to declare.