Abstract
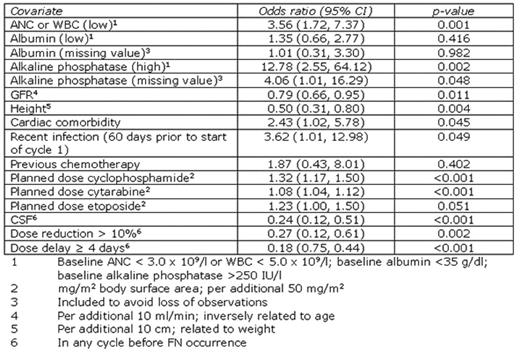
Febrile neutropenia (FN) following chemotherapy often results in hospitalisation and chemotherapy dose delays or reductions in subsequent cycles. Although current guidelines highlight non-Hodgkin lymphoma (NHL) patients receiving standard regimens as having a significant risk of FN, the contribution of additional factors to the overall risk of FN is not fully understood. We present a logistic regression model describing the relationship of various clinical factors with FN occurrence in NHL. Predictors were chosen based on their clinical and statistical significance (5% level) and to allow comparison with other models. Data were obtained from the INC-EU Prospective Observational European Neutropenia Study, which aimed to assess the incidence and predictors of neutropenia, FN and chemotherapy dose limitations in breast cancer and lymphoma. 240 NHL patients were recruited from 37 centres in Belgium, France, Germany, Spain, and the UK. Ann Arbor stages were distributed: I 18%; II 26%; III 17%; IV 40%. Chemotherapy regimens received were 3-weekly CHOP-like (74%), 2-weekly CHOP-like (17%), ACVBP-like (4%) and other regimens (5%). Overall colony stimulating factor (CSF) prophylaxis in patients who had not previously experienced FN was 50%. FN incidence in any cycle was 22%. The resulting logistic regression model of any FN occurrence is shown in the table. Replacing GFR and height with the closely related parameters of age and weight slightly affected predictive ability but did not affect model stability. The area under the receiver operating characteristic curve was 0.84. Using the optimal probability cut-off, model test characteristics were 75% sensitivity and 76% specificity; corresponding to positive and negative predictive values of 48% and 91%, respectively. Low absolute neutrophil or white blood cell count (ANC, WBC), high baseline alkaline phosphatase, low GFR (or high age), low height (or low weight), cardiac comorbidity, recent infection, and high planned chemotherapy dose appeared to increase the risk of FN occurrence. Although chemotherapy dose reduction or delay protected against FN, these are associated with reduced survival in patients receiving CHOP-like regimens. CSF use was a protective factor. These results complement risk factors highlighted in current guidelines.
This study was supported by an unrestricted educational grant from Amgen, Europe.
Author notes
Disclosure:Consultancy: Matthias Schwenkglenks, European Center of Pharmaceutical Medicine, Basel, Switzerland: Amgen. Research Funding: André Bosly, Clinique Universitaire UCL, Godinne, Belgium: Amgen, Belgium; Robert Leonard, Hammersmith Hospital and Imperial College, London, UK: Amgen; Matthias Schwenkglenks: Amgen. Honoraria Information: Ruth Pettengell, St George’s University of London, London, UK: Amgen, Schering, Bayer, Roche; Christian Jackisch, Klinikum Offenbach, Offenbach, Germany: Amgen Europe, Amgen Germany, AstraZeneca Germany; Robert Leonard: Amgen, UK; Matthias Schwenkglenks: Amgen. Paid Export Testimony Information: Ruth Pettengell: Amgen, Schering, Bayer, Roche.