Abstract
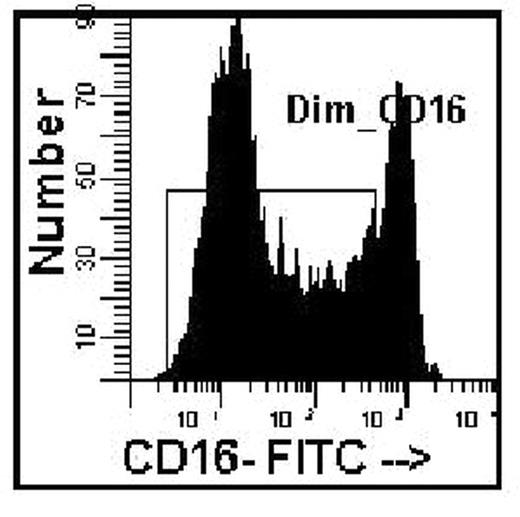
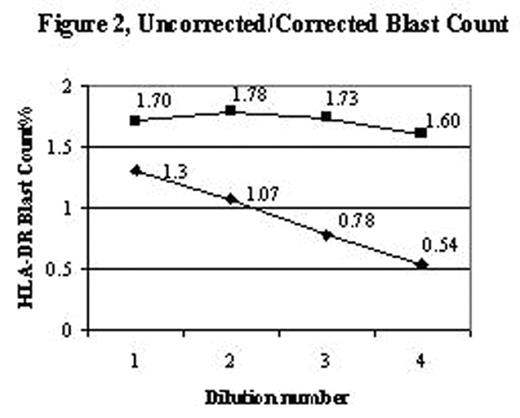
An accurate blast count is pivotal in the diagnosis, classification and prognosis of patients with myelodysplasia. Blast counts in all previous classification schemes are based on morphologic assessment of marrow aspirates with a poor correlation to blast counts determined by flow cytometry. A significant problem in blast enumeration by flow cytometry is the variable hemodilution of the marrow during collection for flow cytometric analysis. Blast counts can vary depending on which aspirate tube is used for flow analysis, e.g., 2.4%, 1st 5ml tube; 0.62%, 2nd 5ml tube; 0.58% 3rd 5ml tube. Morphologists circumvent this problem by selecting a region for assessment close to a spicule with minimal blood dilution. Cell surface antigens can be used to distinguish mature cells found in blood as distinct from immature cells identified in marrow. CD16 intensity on neutrophils reaches a maximum at the band/segmented stage of development with a low coefficient of variation, thereby becoming a marker for mature myeloid forms. A simple method to distinguish immature from mature myeloid cells was developed to assess extent of blood contamination in marrow aspirates using a combination of CD16, CD13, and CD45. The average mature neutrophil content of a marrow was determined from phenotypically normal bone marrow biopsy specimens, assumed to have minimal blood contamination. The proportion of dimCD16 cells gated on the myeloid cells based on CD45 and right angle light scatter in 31 biopsy specimens was 82% (range 69–93, SD=6.2) (Figure 1). A value of 80% (rather than 82%) was used for the subsequent calculations to correct for the excess mature neutrophils found in an aspirate as compared to the biopsies (Corrected Blasts = [80 / % dim CD16 myeloid] x determined blast count). To test this hypothesis bone marrow aspirates were diluted with blood at different ratios to mimic blood marrow hemodilution. Blasts (defined as CD45 dim, low right angle light scatter, HLA-DR positive, CD11b negative) were determined for the various dilutions, then corrected based solely on the proportion of dim CD16 myeloid cells (Figure 2). A marrow from an MDS case was also diluted (1:5 v/v) with blood for comparison. The original marrow contained 80% dim CD16 myeloid cells with a blast count of 9.2%. After dilution, only 12% dim CD16 cells were detected with 1.1% blasts, however upon correction (6.67), the blast count was 7.3%, close to the original determination. This approach may provide for more standardization and consistency in the determination of blast counts in MDS marrow specimens using flow cytometric analysis.
CD16 of marrow myeloid cells.
Uncorrected/Corrected Blast Count
Author notes
Disclosure:Employment: Employed by Hematologics, Inc.