Abstract
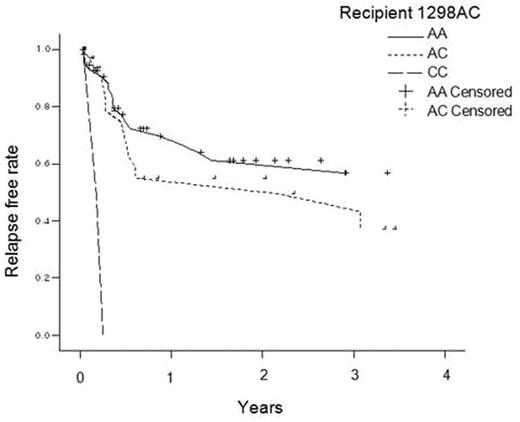
Introduction Clinical significance of polymorphism in MTHFR gene is widely described in various disease entities. Polymorphism is associated with decreased metabolism of methotrexate. Because methotrexate is commonly used to prevent GVHD in HSCT, we studied transplantation outcomes according to genotypes of donor and recipient MTHFR; about the most studied two polymorphism sites (677C/T and 1298A/C). Materials and Methods Patients and donors who underwent allogeneic HSCT due to hematologic malignancy between 1998 and 2006 were retrieved. Patients had received MTX and cyclosporine for GVHD prophylaxis were included. The genotyping of MTHFR polymorphism was screened using SNaPShot Multiplex kit. Clinical data were collected retrospectively. Results We found 99 patients consisted of 60 males (60%) and 39 females (39%) in this homogeneous population. Median age was 34 years (range17–59). Diagnosis were acute leukemia (n=67), chronic leukemia (n=20) and other diseases (n=12). Donor was related(n=72) or unrelated(n=27). Conditioning was myeloablative in 95 patients and nonmyeloablative in 4 patients. Median PFS and OS were 6 (range 0–101) and 15 (range 0–108) months. Clinical factors and genotype are analyzed for risk of hepatic toxicity, recurrence, TRM and death. Peak AST and ALT levels are higher in the 677TT group than in the CC or TT group. In univariate analysis, recipient or donor 1298AA, AC, and CC were likely to relapse in ascending orders (figure1). This was reproduced in multivariate analysis (p=0.008 and 0.011, for each). About TRM and survival, there were no significant correlations with MTHFR genotypes in both univariate and multivariate analysis. Conclusion Simultaneously decreased activity of MTHFR due to 1298AC polymorphism of both donor and recipient might be translated into increased recurrence rate. Validation with randomized trial is required to compare methotrexate to other drug for GVHD prophylaxis.
Author notes
Disclosure: No relevant conflicts of interest to declare.